A Transformative Year

- $18.123M Market Capitalization
Filament Health Corp. (FH.NE) today released its Q4 2021 financial results and has provided operational highlights for the period ended December 31, 2021. Filament’s latest news comes shortly after the Company announced it has been issued a second patent for the extraction and standardization of natural psilocybin and associated psychedelic compounds. For more information, check out this article!
“Since the quarter ended, our efforts have proven productive, including the issuance of a second patent for extraction and standardization of natural psilocybin, the expansion of the Health Canada Dealer’s License held by our subsidiary Psilo Scientific, and Psilo’s inclusion on the Health Canada list of licensed psilocybin producers,” said Ben Lightburn, Co-Founder and CEO.
Financials
According to Filament’s Q4 2021 financial results, the Company’s cash and cash equivalents increased to CAD$4,629,065 as of December 31, 2021, compared to CAD$878,430 on December 31, 2020. In the same period, Filament’s total assets and total liabilities both increased to CAD$17,842,088 and CAD$1,289,530, respectively. For December 31, 2020, these numbers translate to CAD$881,561 and CAD$67,917, respectively.
In total, for the year ended December 31, 2021, Filament reported a net loss of CAD$9,253,607 compared to a net loss of CAD$111,472 on December 31, 2020. The Company’s net loss can be attributed to increased cash used in operations, which totaled CAD$5,407,222 on December 31, 2021, compared to CAD$44,577. The Company’s Q4 disbursement includes an $890,000 payment to UCSF for Filament’s Phase 1 clinical trial.
On October 15, 2021, Filament was listed on the Frankfurt Exchange (FSE) under the symbol FSE:7QS. Additionally, on October 12, 2021, the Company uplisted to the OTCQB Market under the symbol FLHLF and received Depository Trust Company (DTC) eligibility. Keep in mind, Filament listed on the NEO on June 24, 2021, so interpret the Company’s financial result with this in mind. With regards to operational performance, Filament received FDA authorization for a Phase 1 clinical trial.
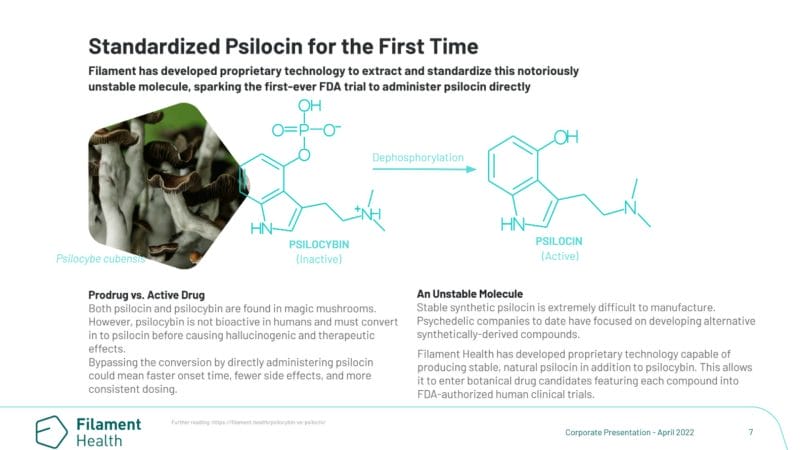
This represents the first clinical trial using naturally-sourced psychedelic substances and the first direct administration of psilocin instead of its prodrug, psilocybin. The Company also entered into a co-development and exclusive licensing agreement with EntheoTech to provide its PEX010 for two of Entheo’s upcoming clinical trials.
If that wasn’t enough, on January 4, 2022, Filament announced that it had partnered with the Canadian Centre for Psychedelic Science for a Health Canada-approved Phase 2 clinical trial utilizing PEX010. It is worth noting that the placebo-controlled trial is expected to begin dosing in the first quarter of 2022.
Share Issuance:
Subsequent to the Q4 2021 period, Filament issued the following shares and options:
- 99,844 common shares to a service provider in exchange for the reduction of $39,939 in debts owing
- 1,650,000 options to purchase common shares to six parties with exercise prices ranging from $0.30 to $0.40 and term to expiry of two to five years
- 226,000 restricted share unit awards, under which the holders have the right to receive an aggregate of 226,000 common shares
- 270,000 common shares upon the exercise of 270,000 options resulting in proceeds of $100,000
Company Highlights

Most recently, Filament announced that it has received its second patent from the Canadian Intellectual Property Office (CIPO). However, subsequent to Q4 2021, the Company has achieved a number of additional milestones. For example, Filament entered into multiple licensing agreements, including agreements with Cybin Therapeutics and ATMA Journey Centers.
“During the fourth quarter, we expanded our partnership network with two licensing agreements, and continued to build our drug development platform and infrastructure,” continued Ben Lightburn.
Additionally, Filament’s wholly-owned subsidiary Psilo Scientific Ltd. has spread like fungi. On January 26, 2022, Filament announced that Psilo Scientific has joined Health Canada’s list of licensed psilocybin producers, marking a significant milestone for the Company. Furthermore, Psilo Scientific received an amendment to its existing Health Canada Dealer’s License enabling the possession, production, research, supply, and delivery of additional controlled substances.
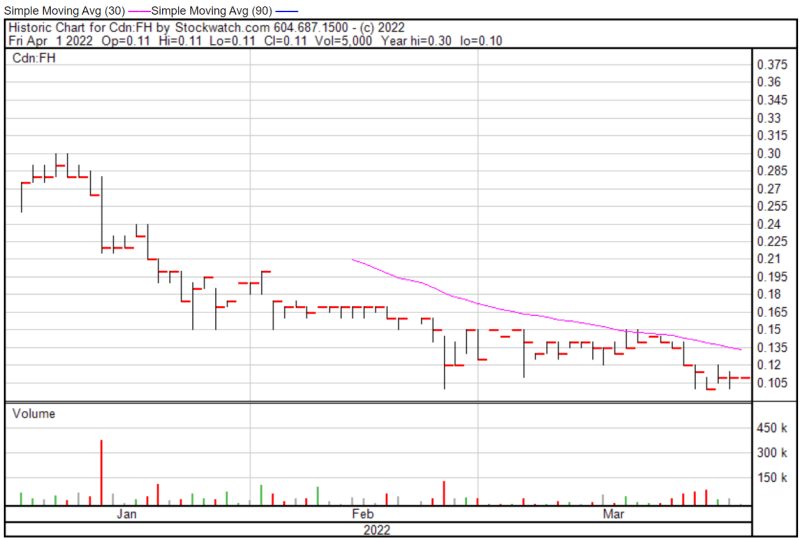
Filament’s share price opened at $0.11 today, compared to a previous close of $0.11. The Company’s shares were trading at $0.11 as of 11:53 AM EST.