In lieu of the American markets not opening today courtesy of the civic holiday, Xphyto Therapeutics (XPHY.C) has elected to provide a glimpse into their business strategy and milestones for 2021.
As an overview, the company sees itself on the edge of a transformational change as their product development programs go from the laboratory to the clinic. Xphyto intends to grow through the commercialization of their existing products and work on focusing investment in areas where they feel the potential for value creation is the strongest.
Vague, I know, but we’ll get there.
“Two thousand twenty was a very productive year for Xphyto. We made significant progress in all areas of our business. We have ambitious milestones for 2021 with multiple product launches on the horizon, multiple clinical drug programs under way and an aggressive commitment to psychedelic medicine. I am extremely confident that our team can execute on the company’s business plan for 2021,” said Hugh Rogers, chief executive officer and director of Xphyto.
The company intends to continue to use its European and North American scientific resources and operations to their best advantage for product development and optimization while expanding both their supply and distribution chains. After 2020, which was by all accounts a successful year for them, they’re looking forward to commercializing their infectious disease diagnostics, completing clinical trials of their transdermal and sublingual drug formulations, and getting deeper into the investment and development of their psychedelic division.
COVID-19
Xphyto’s also been involved in fighting COVID-19 and have milestones set out specifically for continuing this drive. The company’s bread and butter diagnostic product is a PCR (polymerase chain reaction) diagnostic test system, secured under an exclusive commercialization deal with their German development partner, 3a-daignostics GmbH. PCR testing is, at least as of right now, the only international recognized standard for COVID-19 testing and it’s anticipated that it’ll play a role in getting plenty of industries back on their feet in the future, and particularly both domestic and international travel.
Here’s a helpful video to better explain what’s happening:
The PCR system was successfully validated in Q4, 2020, and the company believes that European commercial approval will be reached as of Q1 2021. Xphyto’s in discussions to secure manufacturing and distribution in Europe and the Middle East in time for commercial approval and product launch in Q1, 2021.
Beyond COVID-19 products, though, Xphyto and their partner, 3a, have been working on developing and commercializing a portfolio of oral biosensors. Their lead product is an oral health screening test to detect peri-implantitis, a destructive inflammatory process involving the soft and hard tissues surrounding dental implants culminating in irreversible tissue and bone damage. The company’s milestone for that is late 2021 with European commercial approval and product launch for several screening tests.
Here’s a YouTube clip wherein
Drug Delivery and Psychedelics
In 2020, Vektor Pharma TF GmbH, Xphyto’s German subsidiary, reported developments in four therapeutic development programs. Vektor developed a sublingual (think – under the tongue) formation on contract for a major generic drug manufacturer and distributor.
Now in 2021, the company wants to complete human pilot studies on four products, such as the rotigotine transdermal patch for Parkinson’s disease, a CBD oral or sublingual strip for treatment resistant epilepsy, a THC sublingual for anorexia, and finally, a CBD and THC combined sublingual for spasticity related to multiple sclerosis. The company is presently talking with potential commercial partners, licensors and distributors about how they’re going to get this product to the market.
In terms of psychedelics, Xphyto entered into a few agreements in 2020, including one to develop industrial-scale biotech processes for psilocybin, and secondarily, for research and development related to psychedelic compounds, including mescaline, psilocybin, LSD, MDMA, DMT and others. This year, the company intends on advancing and expanding on these programs, focusing specifically on the industrial scale production of psychedelic API, and launching new programs for the development of drug formulations using psychedelics with their focus being on using their sublingual and transdermal delivery options. The ultimate goal for these are to have products ready to go to established clinical care programs for mental health indications.
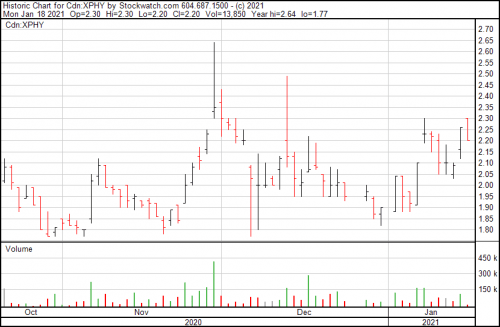
The chart at present only gives some of the story. This company has been engaging in a steady, conscious buildout and are ready for the next stage of their development while they wait for the legislation on psychedelics to catch up with the industry drive.
—Joseph Morton
Full disclosure: Xphyto Therapeutics is an equity.guru marketing client.