Medexus (MDP.T), a Canadian-based specialty pharma firm focused on acquiring and distributing innovative rare disease treatment solutions in North America, announced today that its strategic partner, medac, had resubmitted its New Drug Application (NDA) for treosulfan to the U.S. Food and Drug Administration.
This submission was a response to the FDA request to submit information to complete medac’s April 2022 NDA resubmission and initiate FDA review.
Medac’s NDA requests approval of treosulfan in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT).
In layperson’s terms, allo-HSCT is a procedure which replaces damaged blood-forming cells (stem cells) in a patient who has recently undergone radiation treatment and high-doses of chemotherapy with healthy stem cells from a donor. This treatment is most often used to treat blood cancers like leukemia and lymphoma as well as types of blood or immune system disorders.
Treosulfan is part of a preparative regimen for allo-HCST to be used in combination with fludarabine for treating eligible patients with acute myeloid leukemia and myelodysplastic syndromes.
If the NDA is considered complete by the FDA, the review clock for the NDA resubmission starts as of the date of submission of a complete response.
Ken d’Entremont, Medexus CEO, commented, “We remain excited about the prospect of a treosulfan approval in the United States and about treosulfan’s significant potential in the U.S. market. We are encouraged by the recent publication of the final study results and analysis of the pivotal phase 3 clinical trial of treosulfan conducted by medac, which met its primary endpoint and key secondary endpoints. An FDA approval within a two- to six-month period from the acceptance date would then pave the way for a commercial launch of treosulfan in the United States in the first half of calendar year 2023.”
Mr. d’Entremont continued, “If approved by the FDA, we expect that treosulfan would have a meaningful impact on Medexus’s total revenue. We estimate that the current market-leading product in the United States generated approximately $126 million in peak annual revenue before genericization.”
Dr. Filippo Milano, a physician scientist, mentioned in a June 6, 2022, interview hosted by Medexus, “The experience we’ve had [with treosulfan] has been outstanding so far. I would really like to have this drug available, not just for me, but for all my colleagues.”
The full interview is available through the News & Media section of Medexus’ corporate website.
Medexus’ product portfolio also includes:
- Rasuvo™ and Metoject® – a unique formulation of methotrexate (auto-pen and pre-filled syringe) designed to treat rheumatoid arthritis and other auto-immune diseases.
- IXINTY® – an intravenous recombinant factor IX therapeutic for use in patients 12 years or older with hemophilia (a hereditary bleeding disorder where blood fails to clot to control bleeding)
- Rupall® – an innovative prescription allergy medication
The company also holds the exclusive US and Canadian rights to commercialize Gleolan, an FDA-approved, orphan drug designated optical imaging agent currently used in patients with glioma as an adjunct for the visualization of malignant tissue during surgery.
Medexus reported cash and cash equivalents totaling $10.02 million USD as of March 31, 2022 for a year end revenue total of $76.7 million with $20.3 million of that coming in Q4 2022. There was a net loss of $2.9 million for YE 2022, compared to a net loss of $28.26 million reported for YE 2021.
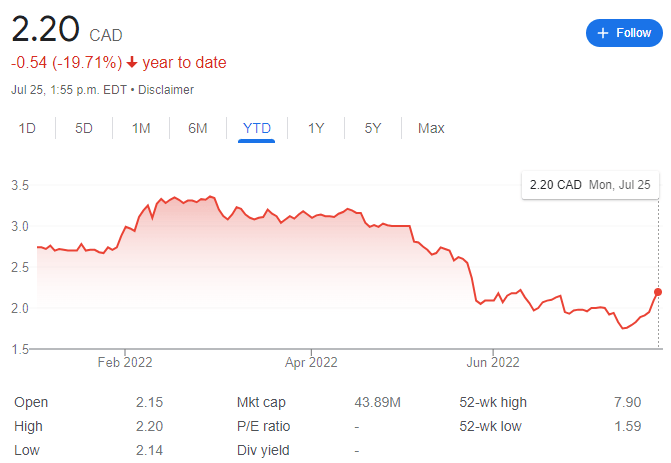
The company currently trades at $2.20 CAD per share with a market cap of $43.89 million.
–Gaalen Engen