Patent Issuance

- $18.123M Market Capitalization
Filament Health Corp. (FH.NE) announced today that it has been issued a patent by the United States Patent and Trademark Office (USPTO) for the extraction and standardization of natural psilocybin and associated psychedelic compounds. The patent covers essential technology for transforming variable psychedelic raw materials into pharmaceutical-grade, standardized drug candidates.
“The issuance of Filament’s first US patent is a testament to the strength of our drug development platform…This approval represents important progress for our intellectual property portfolio,” said Benjamin Lightburn, Chief Executive Officer.
If you have been keeping up with Filament, then you would know that the Company has developed its own proprietary technology capable of producing stable, natural psilocybin and psilocin. With this in mind, Filament has overcome the challenges commonly associated with the production of natural psychedelic compounds, including low yields, instability, and a lack of repeatability.
In general, the process of producing psychedelics on a large scale is daunting. Technological and logistical barriers are especially tricky for a few reasons. In particular mass-producing mushrooms to be easily replicable in large numbers while maintaining their potency and chemical consistency isn’t easy. Pair this with the challenges of natural psychedelics, and you have yourself quite the pickle.
Yet, Filament has overcome these challenges to become the first public company to receive a patent from the Canadian Intellectual Property Office for the extraction and standardization of natural psilocybin and other compounds. With this in mind, Filament currently holds multiple patents, including the Company’s second patent announced on March 23, 2022.
With another patent under its belt, Filament is well-positioned for allowances of several patent-pending applications covering additional elements of its proprietary technologies and compositions. Overall, Filament’s success rate for pending applications is 100%, which is well above the industry standard of 42.8%.
PIND Meeting
Just yesterday, Filament announced that it has held a pre-investigational new drug application (PIND) meeting with the FDA Division of Psychiatry to discuss the Company’s natural psychedelic drug candidates, drug development strategy, and long term plans. Filament also announced that it has received official minutes of the meeting from the FDA. More details along with a virtual update can be found here!
Looking forward, ATMA Journey Centers will begin a trial in mid-2022 utilizing Filament’s PEX010 in order to document the safety of synthetic psilocybin when administered to healthy participants in a controlled clinical setting. Additionally, this trial is intended to provide experience for therapists. If you would like to know more about Filament’s licensing agreement with ATMA, check out this article!
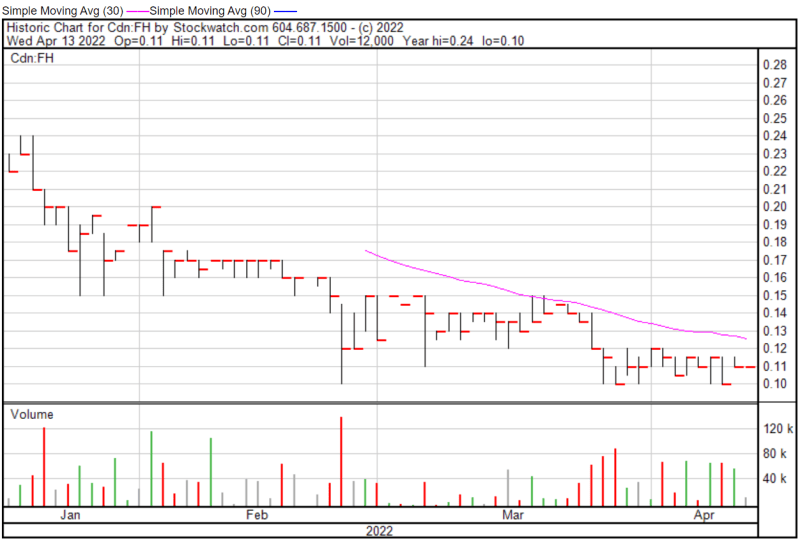
Filament’s share price opened at $0.11 today, compared to a previous close of $0.11. The Company’s shares were trading at $0.11 as of 10:04 AM ET.