Meeting with the FDA
- $18.123M Market Capitalization
Filament Health Corp. (FH.NE) announced today that it has held a pre-investigational new drug application (PIND) meeting with the FDA Division of Psychiatry. During the meeting, Filament and this division discussed the Company’s natural psychedelic drug candidates, drug development strategy, and long-term plans. Filament has received the official minutes of the meeting from the FDA.
“We thank The Division for their time and their valuable insights…The opportunity for biotech companies like Filament to meet with the FDA to discuss pertinent issues in real-time is highly beneficial,” said Jeff Fellows, Head of Regulatory Affairs at Filament Health.
The meeting was attended by FDA senior staff, including numerous medical, product, and nonclinical reviewers. On Filament’s side, the Company was represented by its CEO, Benjamin Lightburn, and Co-Founder, Jeff Fellows. Additionally, Filament’s Ryan Moss, Chief Science Officer, and Joshua Woolley, MD/Ph.D, Clinical Advisor and Director of the Translational Psychedelic Research Program (TrPR) at the University of California San Francisco, were also in attendance.
For context, the Division of Psychiatry (DP) regulates and reviews Investigational New Drug (IND) applications and marketing applications for products for the treatment of psychiatric diseases and conditions such as bipolar disorder, schizophrenia, and obsessive-compulsive disorder, among others.
“We are pleased that the FDA is receptive to our drug development plans…Our conversation was open and productive, and we look forward to many more between Filament and The Division in the future,” said Filament’s CEO, Benjamin Lightburn.
Conference Attendance
Previously, on April 7, 2022, Filament announced that the Company will be participating in a variety of investor conferences, including the Benzinga Psychedelics Capital Conference (BPCC), NobleCon18, and the KCSA Psychedelic Virtual Investor Conference (KCSA PVIC). While the BPCC and NobleCon18 conferences will be held in person, the KCSA PVIC conference will take place virtually.
BPCC Conference: Fontainbleau, Miami Beach, FL; April 19, 2022, Mr. Lightburn will present at 1:30 PM ET.
NobleCon18 Conference: Hard Rock Guitar Hotel, Hollywood, FL; April 21, 2022, Mr. Lightburn will present at 10:00 AM ET.
KCSA PVIC Conference: held virtually on April 28, 2022, Mr. Lightburn will present at 11:30 AM ET.
For more information about the conferences, or to schedule a one-on-one meeting with Filament’s management, those interested are encouraged to contact their appropriate representative directly. Additionally, investors can email KCSA Strategic Communications at KCSA-investor-relations@filament.health.
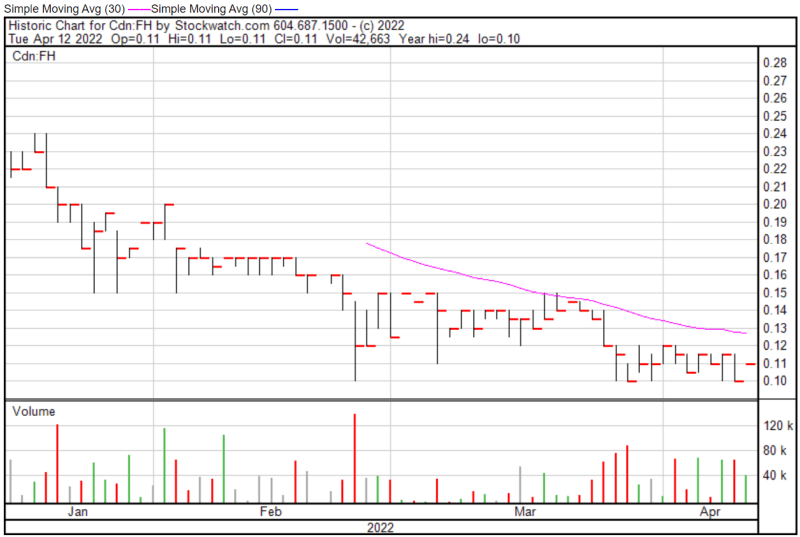
Filament’s share price opened at $0.11 today, compared to a previous close of $0.11. The Company’s shares were up 10% and were trading at $0.11 as of 10:17 AM EST.