Standardizing Psychedelic-Assisted Therapy
- $25.537M Market Capitalization
Filament Health Corp. (FH.NE) announced today that it has entered into a licensing agreement with ATMA Journey Centers. Under the agreement, Filament has licensed PEX010 (25 mg) to ATMA for use in clinical trials, including a Phase I psilocybin safety trial in healthy individuals enrolled in a psychedelic-assisted therapy training program.
“The selection of our natural psilocybin drug candidate for ATMA’s future clinical trials is a validation of both our product and the ease of working with Filament Health…ATMA is a leader in psychedelic-assisted psychotherapy and we are thrilled that they will be using our product,” said Filament Chief Executive Officer, Benjamin Lightburn.
ATMA Journey Centers

ATMA, which translates to inner essence, breath, or soul, is a Canadian company focused on delivering practical, innovative, and transformative experiences leveraging the potential of psychedelic medicine. It is also worth noting that ATMA is Canada’s first private therapy company to conduct legal psilocybin therapy through the Health Canada Section 56 Exemption.
For context, the Health Canada Section 56 Exemption provides physicians, therapists, and people with terminal illnesses access to psilocybin, MDMA, and other psychedelics as an alternative treatment. With this in mind, Health Canada has permitted ATMA to assist Canadians facing end-of-life distress from terminal illness prognoses.
Furthermore, the company utilizes its Therapist Network and Services (TNS) to provide therapists with ongoing support through tools, resources, and training. In doing so, TNS assists therapists in offering psychedelic-assisted therapy without needing to dedicate excessive amounts of time and resources to learning.
Core TNS Services:
- Accredited Training
- Clinical Trials
- Prescription Model
- Medicine Sourcing
- Network Resources
- ATMA Services
- Guide Certification
- Marketing & Referrals
So, how does ATMA actually assist therapists in incorporating psychedelics into their practice? ATMA has partnered with Wayfound Mental Health Group, a Calgary, Alberta-based national center for timely, person-centered mental health care. Additionally, with support from the Psychologists Association of Alberta (PAA), ATMA has developed an 8 week, 20-hour introductory training program for mental health professionals to begin developing competency in providing psychedelic-assisted therapy.
Clinal Trials

As referred to previously, Filament has licensed PEX010 (25 mg) to ATMA for use in clinical trials. These trials are intended to provide therapists with an opportunity to gain in-depth psychedelic-assisted therapy practice, ranging from client preparation to post-integration psychotherapy, via ATMA’s Pilot Program. Getting back to the news at hand, Filament will license PEX010 for various clinical studies, including ATMA’s Phase I psilocybin safety trial, which was recently approved by Health Canada.
To elaborate, on January 7, 2022, ATMA was granted a No Objection Letter (NOL) by Health Canada, representing a crucial step towards the company’s first sponsored clinical trial. Trial participants include licensed health care providers enrolled in a psychedelic-assisted therapy program. The primary goal of the trial will be to assess and document the safety of synthetic psilocybin when administered to healthy participants in a controlled clinical setting.
“Filament’s candidate was an attractive option because they are a licensed manufacturer and their drug candidate has received approval from Health Canada to enter into multiple human clinical trials. We are proud to support a thriving domestic psychedelics industry by selecting Filament’s natural, Canadian-made drug candidate for future studies,” stated David Harder, Co-Chief Executive Officer at ATMA.
Ultimately, based on a growing body of published medical research and first-hand experience, ATMA intends to bring psychedelic-assisted therapy to the clinic. In agreement with Health Canada’s mandate, ATMA and Filament believe the best way to achieve this is through clinical trials demonstrating both safety and efficacy for a variety of mental health indications.
According to L.E.K. Consulting, a global management consulting firm, investment in psychedelics for the treatment of mental health conditions has risen dramatically in the past 12 months. Furthermore, investments are expected to exceed $2 billion, fueled by scientific and clinical results and a growing number of public companies involves in the industry.
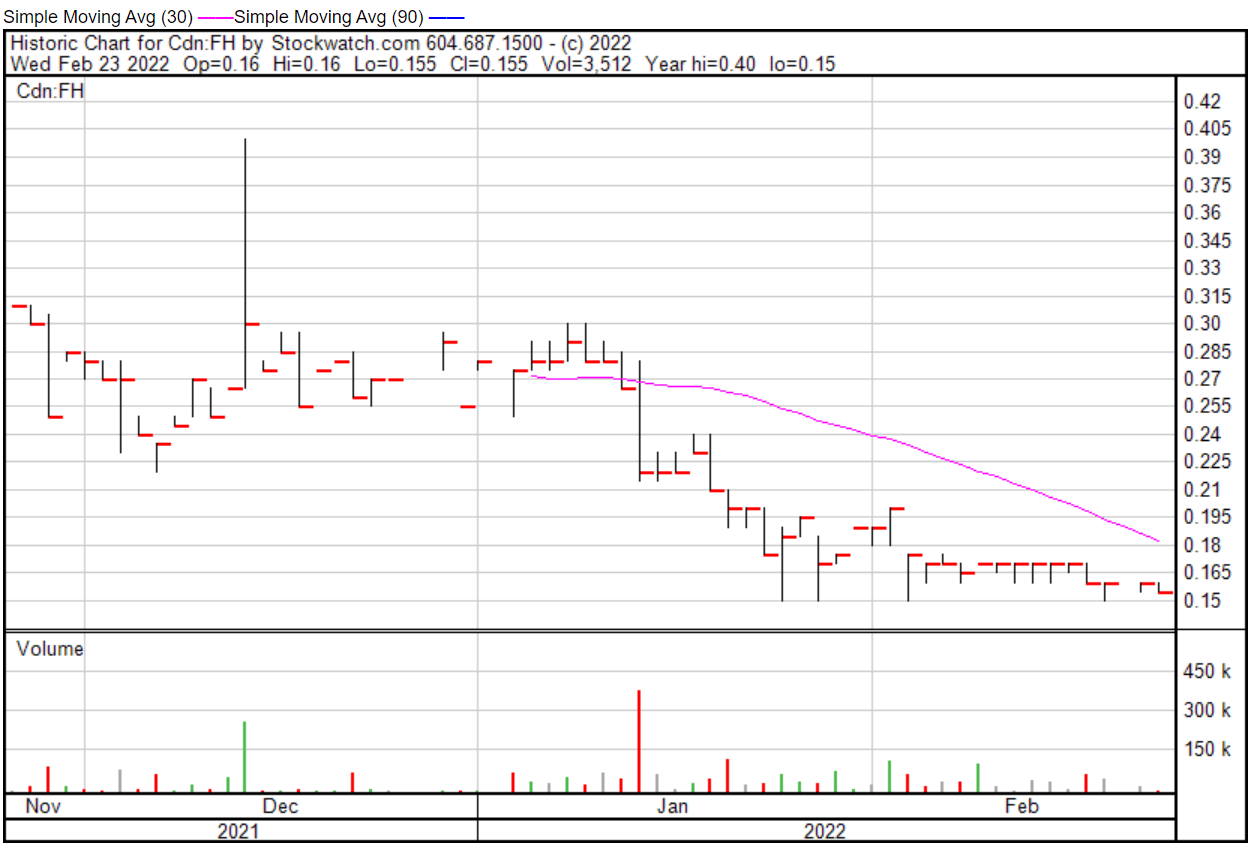
Filament’s share price opened at $0.16 today, even with a previous close of $0.16. The Company’s shares were down -3.12% and were trading at $0.1550 as of 9:34 AM EST.
Full Disclosure: Filament Health Corp. (FH.NE) is a marketing client of Equity Guru.