Medexus Pharmaceuticals Inc. (MDP.T) announced today that the U.S. Food and Drug Administration (FDA) has granted a Type A meeting to medac for treosulfan.
“We have been actively working with medac to prepare for its anticipated Type A Meeting and are pleased that it has been granted this meeting in line with our previously anticipated timeline. We remain positive on the outlook for treosulfan in the United States and look forward to continuing discussions with the FDA in order to meet the requirements for approval,” said Ken d’Entremont, CEO of Medexus.
In case this is your first time hearing about Medexus, let me give you a crash course on the Company. Medexus is an innovative and rare disease pharmaceutical company with a strong North American commercial platform. The Company is focused on the therapeutic areas of rheumatology, auto-immune disease, specialty oncology and pediatrics. Medexus is led by its founder, CEO and President, Ken d’Entremont, a man who has supported the Company since its inception in 2000. Prior to founding Medexus, Mr. d’Entremont was the general manager and vice president of business development at Sanofi, where he led the in-licensing initiatives for Sanofi Canada, a global biopharmaceutical company focused on the development of healthcare solutions from prevention to treatment. Mr. d’Entremont is responsible for leading Medexus to achieve profitability in just its third year of operations.
History Lesson
With this in mind, medac GmbH is a privately held, global pharmaceutical company with a growing pharmaceutical and diagnostic business. The business specializes in the treatment of diseases within the indication areas of oncology, hematology, urology, and autoimmune disorders. On July 12, 2021, Medexus entered into a licensing agreement with medac to commercialize treosulfan in Canada. For context, treosulfan is a bifunctional alkylating agent developed for use as part of the conditioning treatment of patients prior to undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Allo-HSCT is a procedure in which a patient receives healthy stem cells or bone marrow cells from a donor to replace their own. This transplant is most often used to treat blood cancers, such as leukemia and lymphoma as well as certain types of blood or immune system disorders. That being said, treosulfan is used to clear the bone marrow and make room for the transplanted bone marrow cells, which can then produce healthy blood cells.
Prior to receiving a Notice of Compliance (NOC) from Health Canada to commercialize treosulfan in Canada, the Company had been distributing treosulfan through the Special Access Program under the brand name Trecondyv®. Keep in mind, the Special Access Program enables healthcare practitioners to access non-marketed drugs to treat patients with serious or life-threatening conditions when conventional therapies have failed, are unsuitable, or unavailable. In addition to Canada, treosulfan was also granted market authorization in combination with fludarabine, a chemotherapy medication, by the European Commission in June 2019.
Road Block
That being said, Medexus’ treosulfan has since been approved for commercial sale to treat adult patients with Acute Myeloid Leukemia (AML) or Myelodysplastic Syndromes (MDS) who are at increased risk for standard condition therapies, as well as pediatric patients older than one-year-old with AML or MDS. However, Medexus’ plan to commercialize treosulfan in the United States (US) did not go as smoothly as the Company had hoped. On August 3, 2021, Medexus announced that medac, its licensor of treosulfan, had received a Complete Response Letter (CRL) from the FDA.
Long story short, in the CRL the FDA expressed that it could not approve Medexus’ New Drug Application (NDA) for treosulfan in its present form. In other words, without the FDA’s approval, Medexus had to put its plans to launch treosulfan in the US on hold. In the CRL, the FDA also provided recommendations specific to additional clinical/statistical data and analyses pertaining to the primary and secondary endpoints of the completed pivotal Phase III study.
These recommendations were covered by medac’s existing development plan for treosulfan, which the business is contractually responsible to execute and fund. With this in mind, Medexus believes that the CRL provides a path to review and approval that does not require additional clinical studies, provided the Company can satisfy the FDA’s data and post-marketing requirements. If you’re looking for a more detailed explanation regarding the FDA’s CRL, check out this article.
Latest News
As previously mentioned, Medexus announced today that the FDA has granted a Type A meeting to medac for treosulfan. Why is this important? A Type A meeting is necessary for proceeding with a stalled product development program or to address an important related safety issue. With this in mind, a Type A meeting is an important step towards enabling Medexus to commercialize treosulfan in the US.
We continue to believe that treosulfan has enormous potential in the United States, where the current market-leading product, busulfan, reached US$126M in sales prior to genericization. In the meantime, we continue to ramp up sales in Canada, where we commercially launched in September of this year,” continued Ken d’Entremont.
According to Coherent Market Insights, the Global All-HSCT Market was estimated to be valued at USD$2,092.6 million in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 11.2% between 2020 and 2027. It is worth noting that North America is expected to be the most lucrative region in this market due to a rise in the number of hematopoietic cell transplants. Furthermore, the American Cancer Society estimated that around 21,380 new cases of AML and 10,590 deaths from AML occurred in the US in 2016. With this in mind, the increasing prevalence of MDS is expected to create a conducive environment for market growth.
That being said, Medexus has already established itself within the Canadian and European markets through the commercialization of treosulfan. Having now arranged a Type A meeting with the FDA through medac, Medexus is one step closer to commercializing treosulfan in the US as well. According to the Company’s latest press release, the Type A meeting has been scheduled for November 23, 2021.
Financials
According to Medexus’ Q1 2022 Financial Results, the Company had cash and cash equivalents of USD$10,199 thousand on June 30, 2021, compared to USD$18,704 thousand on March 31, 2021. In the same period, Medexus had total assets and total liabilities of USD$142,970 thousand and USD$131,497 thousand, respectively. In total, Medexus reported revenue of $17.3 million compared to $20 million in Q1 2021.
Aside from treosulfan, the Company recently completed enrollment for the Phase IV clinical trial to evaluate the safety and efficacy of IXINITY® in previously treated patients under 12 years of age with hemophilia B. Looking forward, Medexus expects to complete this trial in June 0f 2022. Once completed, this study may support a significant label expansion of the indicated patient population for IXINITY®. For more details related to IXINITY®, check out this article.
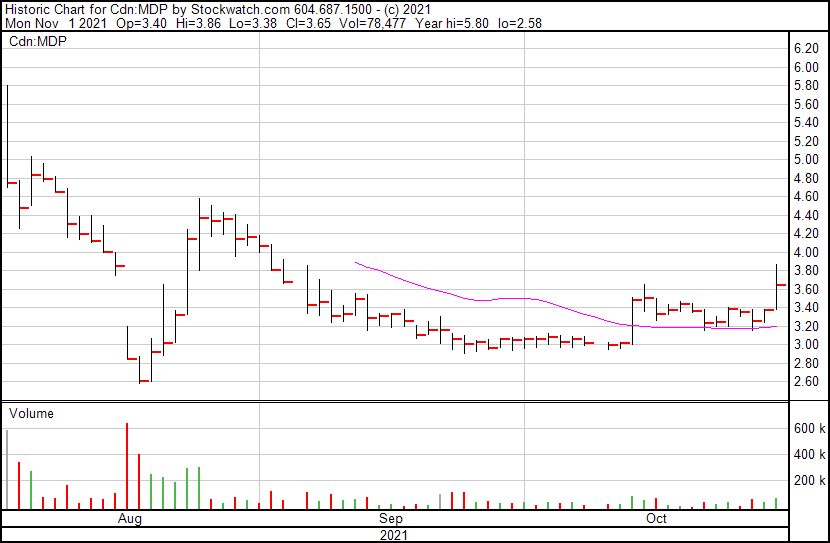
Medexus’ share price opened at $3.40, up from a previous close of $3.31. The Company’s shares are up 10% and were trading at $3.66 as of 1:47 PM ET.







Medexus Pharmaceutical (MDP.T) readies for powerful year of growth in specialty pharma sector