BioVaxys Technology (BIOV.C) announced today that Immunologist Yvelise Barrios, MD, PhD, has joined the Company as a Scientific Adviser to support development of CoviDTH. Furthermore, studies conducted by Dr. Barrios and her colleagues demonstrated that CoviDTH was effective, safe and capable of answering immunogenicity questions in large-scale population.
“The results of Dr. Barrios’ studies infer that our planned US Phase I/II study will likewise find CoviDTH to be effective and safe in humans. This is obviously extremely exciting news for us, as the clinical data from this study has shown that CoviDTH has the potential to answer basic SARS-CoV-2 immunogenicity questions in large-scale populations,” stated Kenneth Kovan, President and Chief Operating Officer of BioVaxys.
Dr. Barrios is a specialist in Clinical Immunology at Hospital Universitario de Canarias, with a focus on histocompatibility, autoimmune diseases and allergy. Furthermore, she is a senior consultant for primary immunodeficiency diseases. She received her PhD on Active Immunotherapy in B-cell Tumors, from Universidad Autonoma, Madrid, and did her post-doctoral in phage display expression of antibody fragments in Immunotechnology at Lund University in Sweden. Needless to say, Dr. Barrios is more than qualified to assist BioVaxys’ development of CoviDTH.
CoviDTH is BioVaxys’ disposable point-of-care diagnostic tool that screens for a T-cell response to SARS-CoV-2 in vaccinated patients or those exposed to SARS-CoV-2. CoviDTH comes as a single-use, disposable, syringe containing the proper amount of SARS-CoV-2 spike protein. Through intradermal injection, the injection site can then be inspected for erythema and induration 24-48 hours later. In other words, if the injection site breaks out in a rash or the area becomes firm, health care providers can visually ascertain the presence of SARS-CoV-2. With this in mind, CoviDTH is an accessible diagnostic tool that does not require extensive medical experience to analyze results.
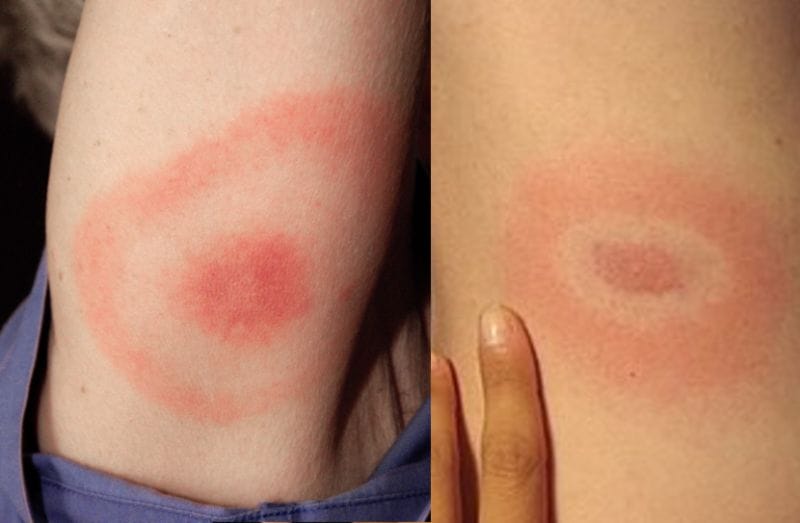
In June, the medical research journals Clinical Immunology and Vaccines both published the results of two clinical studies led by Dr. Barrios and her colleagues. During these studies, Dr. Barrios evaluated the use of DTH reaction to measure cellular immune responses to SARS-CoV-2 in patients after infections, and in individuals vaccinated with the Pfizer and mRNA vaccine. According to study results, the DTH diagnostic approach was both safe and effective.
“The use of this test will provide clinicians with a fundamental tool to answer immunogenicity questions basic to understanding how long T cell immune responses are detectable in exposed and vaccinated individuals. This simple method is also ideal for those groups of patients that do not have easy access to troublesome in vitro studies, such as the young and pediatric population, being extremely useful because it can be interpreted by any non-specialist medical doctor. The study of cellular immune responses in Covid-vaccinated individuals will also provide insight to optimize dosing and type of vaccines in different scenarios of selected groups of patients such as immunodeficient and transplant patients,” stated Dr. Barrios.
In the future, Dr. Barrios and her colleagues will collaborate with BioVaxys on research that explores the use of the DTH reaction to measure cellular immune response in a population exposed to different variants of SARS-CoV-2. As of January 31, 2021, BioVaxys’ total assets were CAD$8,677,504 an its total liabilities were CAD$318,906. Moreover, the Company’s cash at the end of the same period was CAD$857,103, a decrease from CAD$2,423,095 on October 31, 2020. However, this can largely be attributed to an increase in operating expenses, which grew substantially, relative to the Company’s expanding product pipeline. In addition to BioVaxys’ CoviDTH diagnostic tool, the Company is also developing BVX-0918A for ovarian cancer and BVX-0320 for SARS-CoV-2 vaccination.
BioVaxys’ share price opened at $0.245, up from a previous close of $0.225. The Company’s shares were trading at $0.225 as of 11:20AM ET.