BioVaxys Technology (BIOV.C) announced today that the US Food and Drug Administration (FDA) has reviewed its pre-IND request for a Type B review of the Company’s Covid-T™ program.
“We are pleased that our Pre-IND submission package was successful in presenting the rationale for CoviDTH and our development plans. The FDA review and response to our questions will dovetail with the in vivo animal safety study of CoviDTH that we are starting in July and ensure that we have the necessary information to file our IND,” stated Kenneth Kovan, President and Chief Operating Officer of BioVaxys.
The FDA has determined that a written response is sufficient to address BioVaxys’ questions regarding bioproduction and clinical development plans for its planned pivotal Phase III study, which includes Covid-T™, the Company’s T-cell immune response diagnostic for SARS-CoV-2. The FDA has stated that this written response will be available by July 23, 2021. For context, pre-IND refers to “pre-investigational new drug” (IND). With this in mind, submitting a pre-IND request indicates that a company would like feedback on its IND development program.
The pre-IND meeting represents a critical point in the regulatory process and provides companies with an invaluable opportunity to receive FDA feedback, while establishing a positive relationship with the agency. A pre-IND meeting can provide a multitude of benefits, most prominent being a reduction in the time to market for an IND. Furthermore, FDA feedback can help a company bring their IND to market faster by helping said company identify and avoid unnecessary studies, define endpoints and goals, and minimize costs.
With this in mind, BioVaxys submitted a pre-IND meeting request and briefing package with the FDA’s Center for Biologics Evaluation and Research (CBER) for Covid-T™ in March earlier this year. Considering the FDA has the option to not grant a pre-IND review for reasons such as it being premature, or not providing adequate basis for review, things are looking good for BioVaxys’ proposed Covid-T™ program.
Covid-T refers to BioVaxys’ low cost, disposable, diagnostic to identify T-cell immune response to the presence of SARS-CoV-2. Covid-T™ is based on Delayed-Type Hypersensitivity (DTH) technology, hence why the product is also referred to as CoviDTH. With this in mind, DTH is known to be a measure of T-cell immunity has been used for many years in immune response screening for other infectious diseases like tuberculosis, fungal diseases, and mumps.
So how does it work? The Covid-T™ test is performed by injecting a small amount of test material, in this case the SARS-CoV-2 spike protein, intradermally and inspecting the site for a reaction 24-48 hours later. To clarify, SARS-CoV-2 spike proteins are harmless on their own, and are even used in vaccines like mRNA. For a simple breakdown of how spike proteins generally work, check out this video from the Children’s Hospital of Philadelphia. By adding the spike proteins from SARS-CoV-2 variants, Covid-T™ has the potential to detect T-cell responses to new mutated variants that are spreading worldwide.
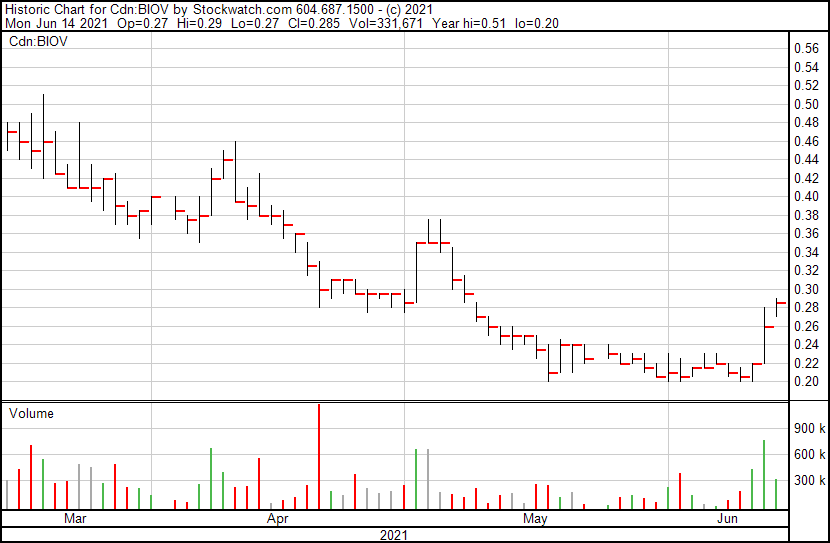
BioVaxys’ share price opened at $0.27, up from a previous close of $0.26. The Company’s shares are up 9.62% and are currently trading at $0.285. This indicates that there has been noticeable change following the news.