BioVaxys Technology (BIOV.C) announced today that the US Food and Drug Administration (FDA) has provided its official Written Response to the Company’s request for a Pre-IND Type B review of CoviDTH as a diagnostic for evaluating T-cell immune response to SARS-CoV-2.
“With the guidance we received from this FDA review, BioVaxys is now able to begin preparing its IND…He adds “Although the FDA has indicated that our planned animal tox study is discretionary, we will likely continue with the animal tox study of CoviDTH as it does not interfere with the development time frame and may in fact provide useful data,” stated BioVaxys President and Chief Operating Officer Ken Kovan.
According to the Written Response, the Chemistry, Manufacturing, and Controls as well as other elements of BioVaxys’ clinical development program, were deemed acceptable by the FDA. Additionally, the FDA has provided guidance and feedback supportive of the Company’s clinical development plans for CoviDTH. Included in BioVaxys’ development plan is a preclinical toxicity study for CoviDTH. On March 15, 2021, BioVaxys and Bioanalytical Systems (Inotiv), a leading Contract Research Organization (CRO), entered into an agreement to conduct preclinical toxicology studies for CoviDTH. Under the terms of the agreement, Inotiv is expected to evaluate the safety, tolerability, and toxicity of a purified SARS-CoV-2 s-protein, a core element in BioVaxys’ CoviDTH.
With this in mind, the FDA has indicated that BioVaxys’ animal toxicity study for CoviDTH is no longer necessary. Additionally, the FDA has given the Company the green light to begin its clinical development program with a combined Phase I/II human study. However, being a studious bunch, BioVaxys’ intends to continue its toxicity studies regardless, with the hopes of uncovering useful data.
“We are pleased to advance CoviDTH towards clinical trials, as we believe that mass screening for T cell immunity to Covid-19 will represent a critical tool for public health authorities to address the continued pandemic, as Covid variants continue to circulate and major governments in the southern hemisphere enact new lockdown policies,” commented James Passin, BioVaxys CEO.
The Pre-IND review represents a critical step in the US regulatory approval process, as it enables study sponsor companies to receive invaluable guidance from the FDA regarding clinical trial design, clinical materials manufacturing, and quality controls, to name just a few. Based on FDA feedback, BioVaxys will begin preparations of an IND application to support a Phase I/II safety, dosing and efficacy study.
According to BioVaxys’ Q1 2021 financial results, as of April 30, 2021, the Company’s total assets and total liabilities were CAD$8,947,298 and CAD$355,554, respectively. Furthermore, for the six months ended April 30, 2021, the Company’s cash grew substantially year-over-year from CAD$167,930 to CAD$1,379,967. With this in mind, BioVaxys is a dirt cheap stock that may be worth keeping an eye on as the Company approaches the commencement of its CoviDTH human clinical trials. If commercialized, CoviDTH could become an invaluable asset for BioVaxys.
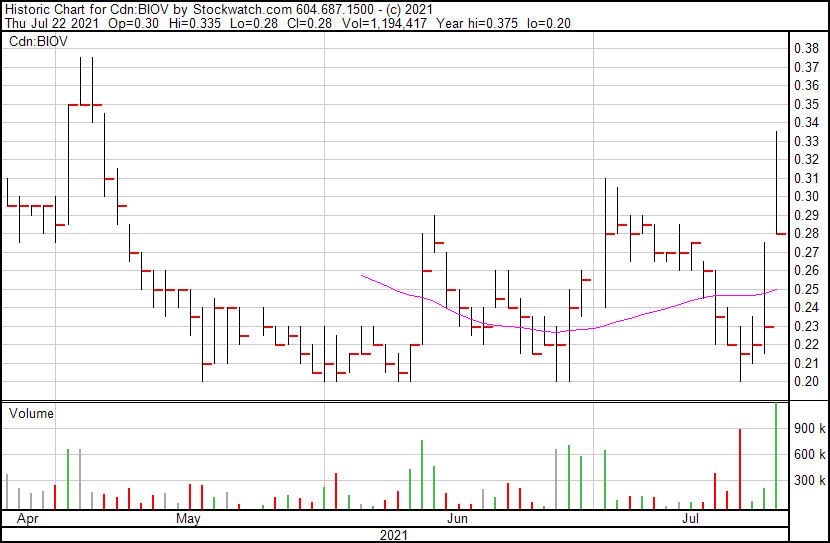
BioVaxys’ share price opened at $0.30, up from a previous close of $0.23. The Company’s shares are up 26% and are currently trading at $0.29 as of 10:11AM ET. This indicates that there has been significant change following the news.