Valeo Pharma (VPH.C) announced that they have begun commercialization of their Enerzair Breezhaler and Atectura Breezhaler following the shipment of the product across Canada and the deployment of their national respiratory sales force.
Valeo signed a distribution deal with Novartis Pharmaceuticals Canada back in March, making them responsible for medical and commercial activities of Novartis’s two asthma therapies, Enerzair Breezhaler and Atectura Breezhaler.
Valeo signed a distribution deal with Novartis Pharmaceuticals Canada back in March, making them responsible for medical and commercial activities of Novartis’s two asthma therapies, Enerzair Breezhaler and Atectura Breezhaler.
Enerzair Breezhaler is provided in a transparent capsule that allows patients to see that they have taken their medication and is https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered via the dose-confirming Breezhaler device, which enables once-daily inhalation using a single inhaler. Enerzair Breezhaler was approved by Health Canada last July.
The Atectura Breezhaler functions in a similar fashion but uses a different formulation.
“We are very excited to start promoting and selling our first respiratory products. The establishment of our country wide respiratory, medical and commercial infrastructure is well under way and it is driven by a team of highly skilled and experienced pharma professionals. Nelly Komari, a seasoned pharma executive, has recently joined Valeo to lead the medical team in support of our asthma product launches. Enerzair Breezhaler and Atectura Breezhaler have demonstrated, against the current standard of care, that they improve the level of control of asthma symptoms and better prevent the related complications. Our team is very excited to start to reach out to clinicians to introduce both products and is proud to provide Canadian patients with these new treatment options,” commented Frederic Fasano, President and COO.
The Asthma market is large, with almost 4 million Canadians suffering from the ailment, where per capita sales of combination inhalers for asthma are among the highest in the OECD. In 2018, sales of asthma combination inhalers alone totalled $577 million in Canada, which accounts for 2.3% of the total pharmaceutical market.
The Asthma market is large, with almost 4 million Canadians suffering from the ailment, where per capita sales of combination inhalers for asthma are among the highest in the OECD.
In places like the US, rates of asthma are increasing each year, meaning we can expect the asthma treatment to grow in the coming years.
“The commercial launch of Enerzair Breezhaler and Atectura Breezhaler is a key milestone for Valeo. It marks the debut of the Company’s commercial efforts in the $700M Canadian asthma market, one of Canada’s largest therapeutic segments,” stated Steve Saviuk, Valeo’s CEO. “I am especially proud of our Valeo team that organized this major product launch less than three months after licensing the products from Novartis Pharmaceuticals Canada Inc.. The commercialization of these two innovative asthma therapies follows the successful launch of RedescaTM in April 2021 and will immediately impact our financial performance and accelerate in the fiscal quarters to come.”
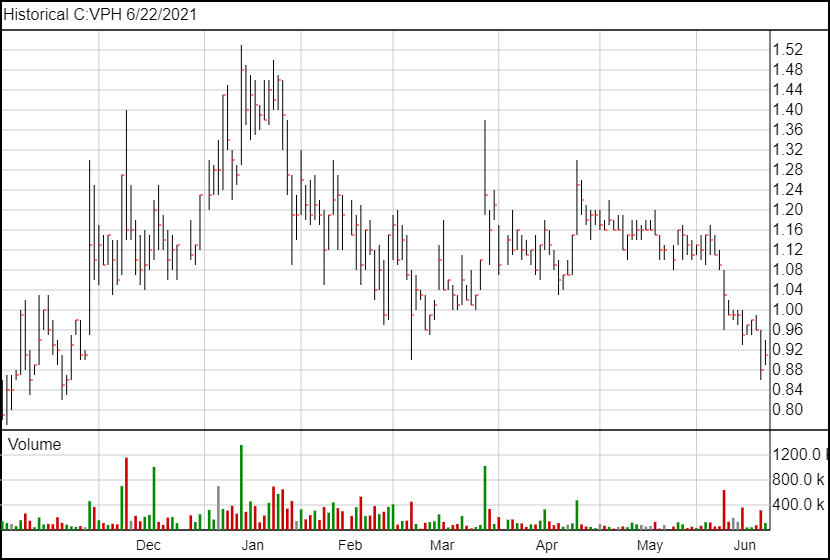
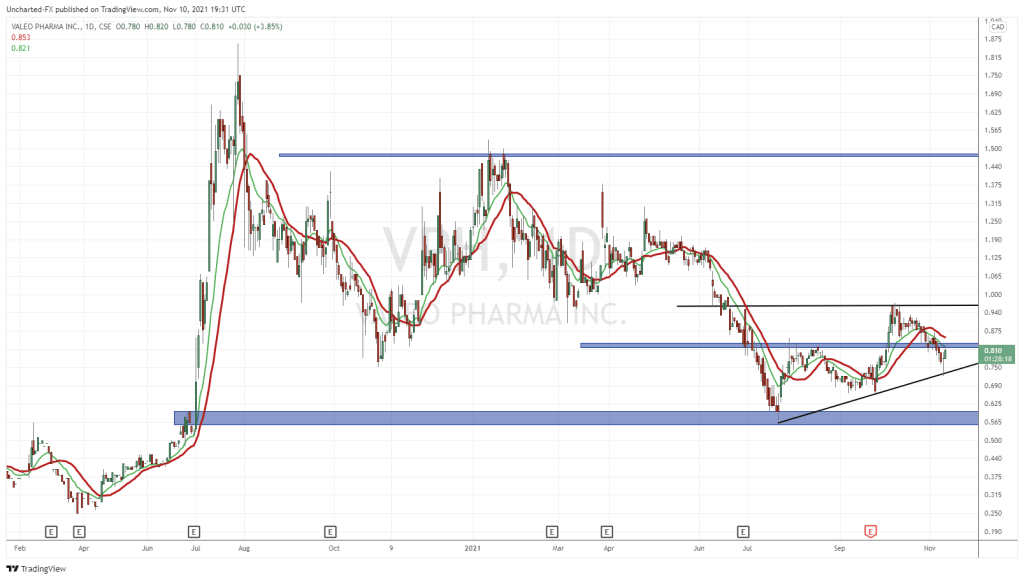
Following today’s announcement, shares of Valeo are up 3 cents and are currently trading at $0.91.
Full Disclosure: Valeo Pharma is an Equity Guru marketing client.