Cybin (CYBN.NE) announced they had completed their 20th pre-clinical study and are progressing their CYB003 and CYB004 proprietary psychedelic molecules into studies that will enable them to be tested as Investigational New Drugs (INDs).
Cybin, along with its partners, has completed key research studies, both in vitro (a term which describes studies done outside of living organisms) and in vivo (which refers to studies done with or within a living organism). The 20 studies were completed on proprietary Cybin technology in record time.
CYB003 and CYB004 arose from these studies as promising development candidates, and have now entered full IND-enabling studies in preparation for future clinical testing.
“Excellent team-work and fully supportive partners have greatly facilitated the advancement of these two new therapeutic candidates with enhanced and improved properties. We look forward to rapid progress towards clinical studies,” stated Michael Palfreyman, Cybin’s Chief R&D Officer. “The pre-clinical studies of CYB003 and CYB004 candidates included API Synthesis and optimization to demonstrate that these two psychedelic molecules show significant in vivo modifications of pharmacokinetics (“PK”) consistent with “Proof of Concept.”
(API Synthesis stands for Active Pharmaceutical Ingredient, and is a topic Equity.Guru has covered before.)
Cybin’s work has led to many discoveries, including over 50 proprietary psychedelic molecules, as well as a handful of delivery mechanisms and supportive technology platforms. CYBN explores the therapeutic potential of a variety of psychedelic-based treatments, including those involving psilocybin, DMT, and MDMA. They alter the drug’s pharmacokinetic profiles (the movement of the drugs in and out of the user’s body) to optimize them for therapeutic circumstances, by providing them with shorter action periods and by reducing side effects.
The legal psychedelic drug market is expected to grow with a CAGR of 16.3% over the next 6 years, and with its increasing inventory of treatments, Cybin could be poised to take full advantage of this market growth in the coming years. Any growth is dependent on the progression of studies, like the ones announced today, which will allow CYBN to eventually perform clinical studies and hopefully make it to market.
Being able to progress to IND-enabling studies in a timely manner is a key part of that process.
“Our internal research and development team, along with an extensive network of partners, has progressed CYB003 and CYB004 into IND-enabling studies at an impressive pace. I am in no doubt we have the best team in this sector and the right team to progress these exciting future treatments into clinical studies over the next 12 months,” stated Doug Drysdale, Chief Executive Officer of Cybin.
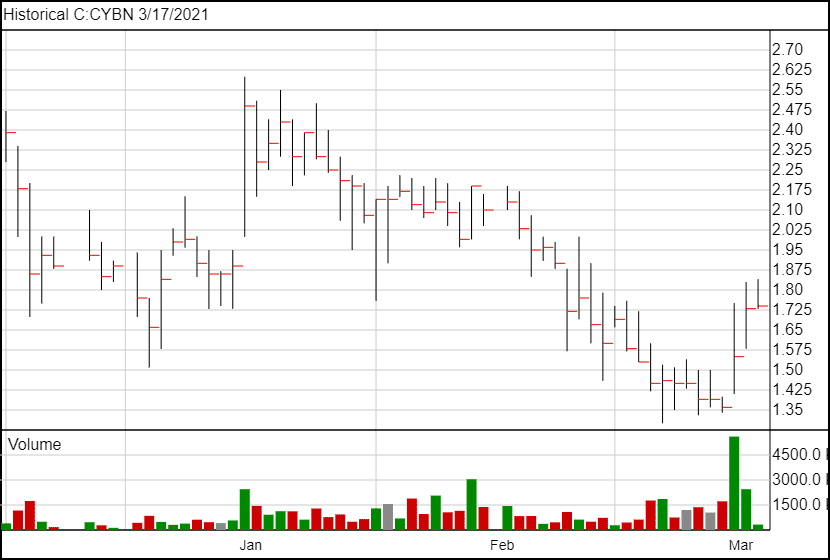
The stock is up 3 cents following the news and is currently trading at $1.76. Over the past week, CYBN.NE has risen nearly 30%.
Full disclosure: Cybin is an Equity.Guru marketing client.