Mydecine Innovations Group (MYCO.C) expanded their relationship with Applied Pharmaceutical Innovation (API) to include the University of Alberta in support of multiple drug development and clinical trials, according to a press release.
The expanded partnership with the University of Alberta and API enhances research capacity and drug development from natural products, as well as novel drug development. The company can already legally cultivate, extract, import and export, as well as commercialize full cGMP pharmaceutical grade natural and synthetic compounds to other licensed facilities across the world.
“Through their work with us, Mydecine has access to tens of millions of dollars of research infrastructure at our facilities across Alberta, and the capability to conduct an extremely robust drug development program. One of the largest barriers for earlier stage companies as they grow is building true commercial R&D capacity, particularly in a quickly moving sector under their agreement with API Mydecine has this in droves and the ability to scale their many programs, pursuing research and development at the cutting edge of this rapidly emerging field,” said Andrew MacIsaac, CEO of Applied Pharmaceutical Innovation.
Mydecine Innovations Group is a biotech and life-science company involved in finding new and novel solutions to treating mental health problems. Their medical and scientific advisory board is working on developing their research and development pipeline of naturally sourced psychedelic-based therapeutics, therapy protocols and delivery systems. The company has access to a completely cGMP certified manufacturing facility that enjoys all of the necessary Health Canada licensing to be able to go from analysis to cultivation to extraction to import and export of active psilocybin compounds.
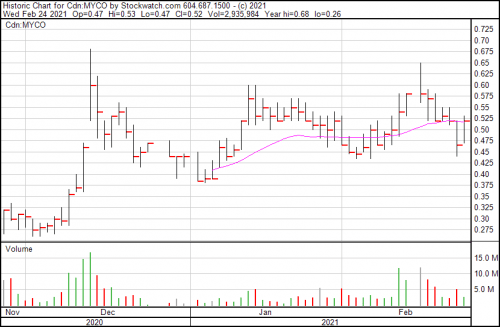
MYCO is up 11.8% today, and now trading at $0.52.
—Joseph Morton