BioVaxys Technology (BIOV.C) announced today that it that it has initiated the clinical trials for Covid-T, the company’s diagnostic platform for detecting T-cell activity, according to a press release.
The US Food and Drug Administration (FDA) has given BioVaxys the nod to file for a pre-Emergency Use Authorization (EUA) for Covid-T. An EUA is one of those little hidden loopholes the FDA has to allow the use of unapproved medical products, or unapproved uses for approval medical products in an emergency to diagnose, treat or prevent serious or life-threatening diseases when certain criteria have been met and there’s a dearth of alternatives.
“We believe that our low cost, scalable, easy-to-https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister test for T cell immunity to SARS-CoV-2 may help solve the urgent global public health crisis of prioritizing the distribution of Covid-19 vaccines; we look forward to rapidly advancing Covid-T™ towards commercialization,” said James Passin, CEO of BioVaxys.
Vancouver-based, VioVaxys is an early stage biotech company in the business of development platforms to fight viruses and cancer, as well as performing immunodiagnostics. The company is pushing forward their own COVID-19 vaccine to fight the SARS-CoV-2 virus that causes it on its viral protein technology, and at present planning a clinical trial dealing with its cell vaccine.
The case for COVID-T is the case of a low-cost, user-friendly tool capable of accurately testing for the presence of T-cells, which could theoretically offer lasting protection against SARS-CoV-2. It’s also been contended that detecting T-cells could potentially assist with the identification of safe and at-risk populations, while providing an ability to evaluate the effectiveness of any vaccines in stimulating T-cell immunity.
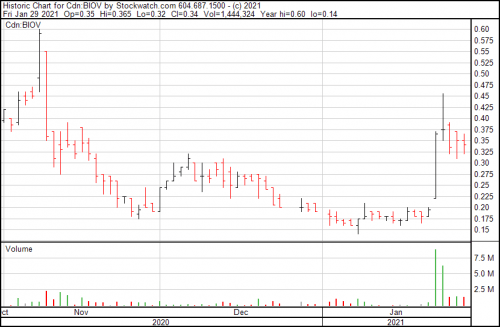
Biovaxys has enjoyed an excellent month, climbing to a double since mid-January. Biovaxys closed today now at $0.34.
—Joseph Morton