Canntab Therapeutics (PILL.C) has received approval for an export license from Health Canada.
“We are delighted to have received Health Canada’s export approval to enable us to meet our Australian partners’ orders in the immediate future. This is the first export order for us and we look forward to many more orders, not only from Cann Global, but from other international partners as they recognize the unique patented product offerings of Canntab…,” said Larry Latowsky, CEO of Canntab.
Canntab is a Canadian biopharmaceuticals company focused on manufacturing and distributing hard pill cannabinoid formulations in a variety of doses and time-released combinations. The Company is recognized for creating cannabinoid and terpene blends in hard pill form for therapeutics applications. With this in mind, Canntab is responsible for providing its hard pill formulations to doctors, patients and consumers with medical grade solutions. The Company’s products incorporate all of the features one would expect from over the counter prescription medication sold in pharmacies globally. This will include Canntab’s extended release and once a day formulations, both intended to provide accurate dosing and improved shelf life.
Although the issue has been recognized, there is still an inconsistency between practitioners and the daily amount of dried cannabis they are prescribing to patients. This is largely due to a lack of understanding or knowledge regarding cannabis as a prescription. Moreover, inhaled cannabis is harder to regulate than hard pill formulations. Compared to dried cannabis, hard pill prescriptions offer consumers a consistent and reliable dosage.
Following the acquisition of an export license from Health Canada, Canntab is now able to fulfill the purchase order received from Cann Global, an Australian company with an influence in the hemp and medical cannabis industry. The initial purchase order of $406,200 includes 6 of Canntab’s SKUs and are expected to be distributed throughout Australia to medical distributors, including doctors, pharmacies, and hospitals.
“…Our order to Australia also enables us to participate in one of the largest medical cannabis studies being conducted globally, and furthermore, we have engaged with Cann Global to begin the necessary work and development to undertake clinical blood studies specifically for measuring the efficacy of our immediate and extended-release products, which we hope to conclude by the end of the summer, our third quarter,” continued Mr. Lotowsky.
Additionally, Cann Global is expected to make Canntab’s products available to the Cannabinoid Medicine Observational Study (CMOS). CMOS intends to collect data from 20,000 participants nationwide with the hopes of assessing the safety and efficacy of medicinal cannabis products for a range of conditions like PTSD, epilepsy, chronic pain and more. With extensive knowledge related to cannabis delivery systems, Canntab’s hard pill formulations will likely generate positive results through CMOS’s study. In doing so, Canntab hopes to lead the transition into what it refers to as ‘Cannabis 3.0’. If hard pill formulations become standardized in the medicinal cannabis field, Canntab is positioned well to lead the market.
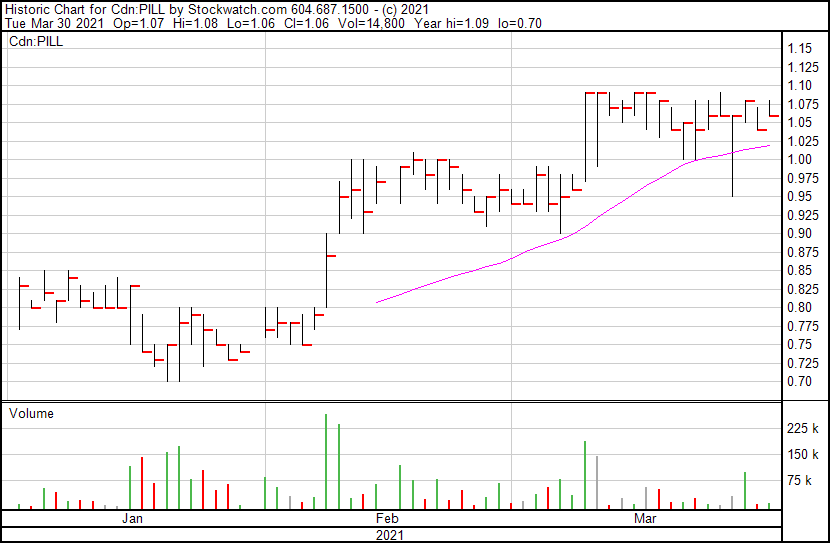
Canntab’s stock price opened at $1.07, up from yesterday’s close of $1.04. The stock price currently sits at $1.06.
Disclaimer: Canntab is a client of Equity Guru.








Do you have any update to share on Canntab? There are no press releases and stock is tanking. Cheers.
Since Canntab’s Co-Founder & President, Jeffrey Renwick, passed away, the Company has been radio silent. Shares have dropped slowly but steadily, likely due to the lack of press releases. If you look at a board like CEO.CA, you’ll see that investors haven’t talked about Canntab since July following the company’s last press release. If Canntab rebounds, we will be sure to cover it!