Canntab (PILL.C) has been granted its 3rd patent by the Australian Patent Office. The patent was granted in relation to the Company’s proprietary cannabidiol formulations with a priority date of January 23, 2017. The patent’s terms expire on January 22, 2038. In addition to receiving its 3rd patent, Canntab also received a $406,200 order in Australia.
“The allowance of Canntab’s patent by the Australian Patent Office continues to further provide fundamental intellectual protection for Canntab’s innovative tableting technology and even further validates the years of research and development the Company has conducted. The Company now has patent protection in the USA, Canada and Australia providing an international scope to the Company’s expanding portfolio of issued patents,” said Gavin Bogle of Magyar, Bogle & O’Hara, Canntab’s Legal Counsel on intellectual property
Aside from the Company’s three granted patents, Canntab also has eleven pending patent applications internationally. With this in mind, Canntab has developed both patented and patent pending technologies. These technologies were created to assist the delivery of standardized medical cannabis extract in various extended and sustained release pharmaceutical grade delivery systems. With its experience in the industry, Canntab is confident that its hard pill formulations are superior to other medical delivery systems that are widely available in gel capsules.
Canntab also received an initial purchase order in the amount of $406,200 with its Australian partner, Cann Global. The initial order includes 6 SKUs of the Company with varying ranges of THC and CBD content depending on the SKU.
Cann Global has received its import license from the Australian Government Department of Health. However, Canntab is waiting for an Export License approval from Health Canada which will enable the Company to ship the order. Shareholders will be updated upon the approval of the Export License.
“These are 2 major milestones that confirms our proprietary formulations are unique and different from other product offerings in the global marketplace. Intellectual property is at the root of our value proposition and having been granted our first Australian patent and our third patent overall in less than 5 months further confirms our leading position as the go to company for precise dosage and pharmaceutical grade tablets and caplets for the medical market…we are completing production of our first order and are in the final stages of approval with Health Canada for the proper export permits that will allow us to ship our order to Australia…we are quite confident that our partnership with and supply to Cann Global will contribute to our planned growth,” said Larry Latowsky, CEO of Canntab
In addition to attaining its 3rd patent and a sizeable initial order, Canntab will be participating in Australia’s largest observational medical cannabis research study. The study will be conducted by Applied Cannabis Research (ACR). Canntab’s products will be used during the duration of the study in collaboration with major Australian clinics and hospitals. The Cannabinoid Medicine Observational Study (CMOS) will gather data from 20,000 patients nationwide over 5 years with results being released on an ongoing basis. Ultimately, CMOS aims to assess the safety and efficacy of medical cannabis products to treat a range of refractory conditions.
Canntab is certainly confident in its ability to develop leading edge medical delivery systems, however, the Company’s confidence is justified. Recent news has further solidified its position as a leading company in the industry.
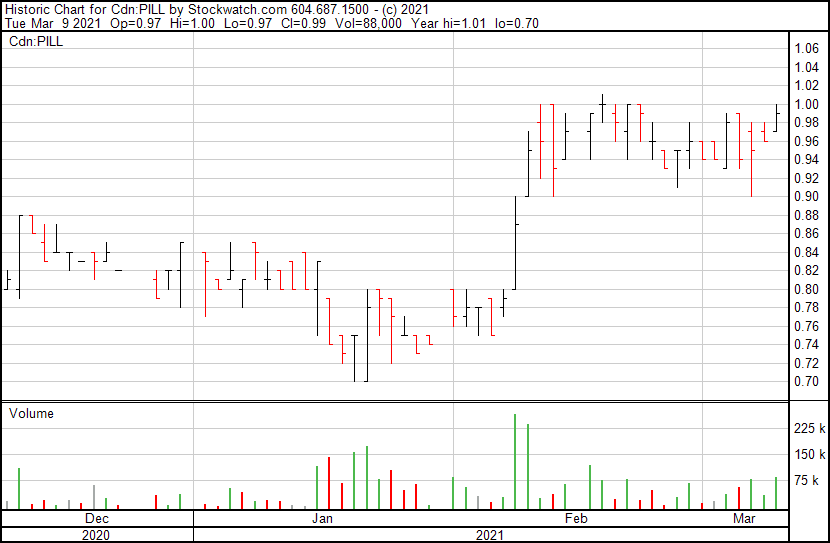
Canntab’s stock price opened at $0.97 and reached a high of $1.00. The stock’s price currently sits at $0.97.
Disclaimer: Canntab is a client of Equity Guru.