Two for Two

- $23.066M Market Capitalization
Filament Health Corp. (FH.NE) announced today that it has been issued a second patent by the Canadian Intellectual Property Office (CIPO) for the extraction and standardization of natural psilocybin and associated psychedelic compounds. The patent covers essential technology for transforming variable psychedelic raw materials into pharmaceutical-grade, standardized drug candidates.
“Two years ago, the conventional wisdom was that producing shelf-stable psilocin was impossible, Not only has Filament proved the contrary, but we have also had our innovations yet again validated by the patent office. The success rate for pending applications to issued patents in the pharmaceutical industry is 42.8%. Our success rate is 100%,” said Taran Grey, Director of Intellectual Property.
Prior to Filament’s latest patent approval, on August 3, 2021, the Company was awarded the first-ever patent for the extraction and standardization of natural psilocybin. With this in mind, let’s talk about Filament’s technology. In addition to securing two patents, the Company also operates one of the first good manufacturing practice (GMP) facilities in the world to also have a Health Canada Dealer’s License. Here, Filament conducts a variety of tasks including:
- propagation
- extraction
- production
- distribution
- sale
Furthermore, Filament’s drug development platform has led to the discovery of important methods of natural extraction and purification. As opposed to synthesizing new molecules, the Company has developed the technology needed to create pharmaceutical-grade drug candidates. These drug candidates are comprised of compounds that already have documented safety and efficacy, thereby increasing speed to market. So, how does it actually work? See for yourself!
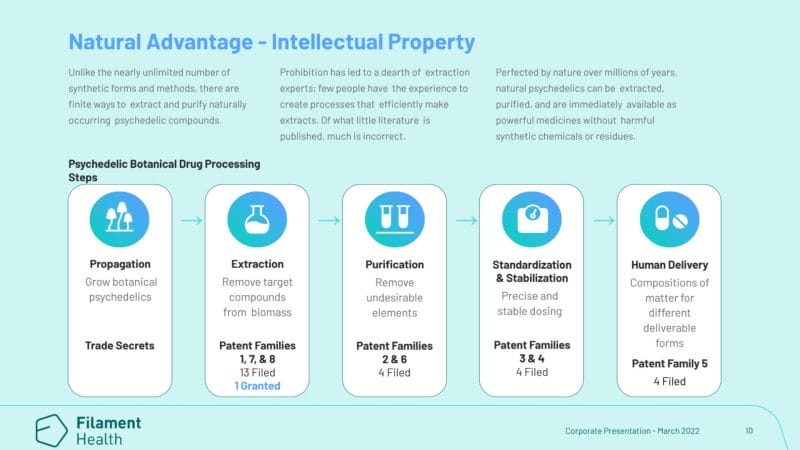
Filament’s latest successful patent issuance validates the Company’s IP strategy and sets the Company up for allowances of several pending patent applications covering additional elements of its proprietary technologies and compositions. As reference by Tarran Grey, the success rate for pending applications to issued patents in the pharmaceutical industry is 42.8%, according to the Yale Journal of Law & Technology.
“The issuance of Filament’s second patent is a testament to the strength of our drug development platform and our grasp of crucial technologies…Valuable medicines can be found in nature and we have built a powerful platform with the ability to transform variable natural substances into a standardized pharmaceutical-grade product,” said Benjamin Lightburn, Chief Executive Officer.
With this in mind, against the odds, Filament is two for two so far, with almost 30 additional patents in the pipeline. It is also worth noting that Filament recently entered into a licensing agreement with ATMA Journey Centers. According to the agreement, the Company will license PEX010 (25 mg), one of its naturally-extracted drug candidates, to ATMA for use in clinical trials. For more details, check out this article!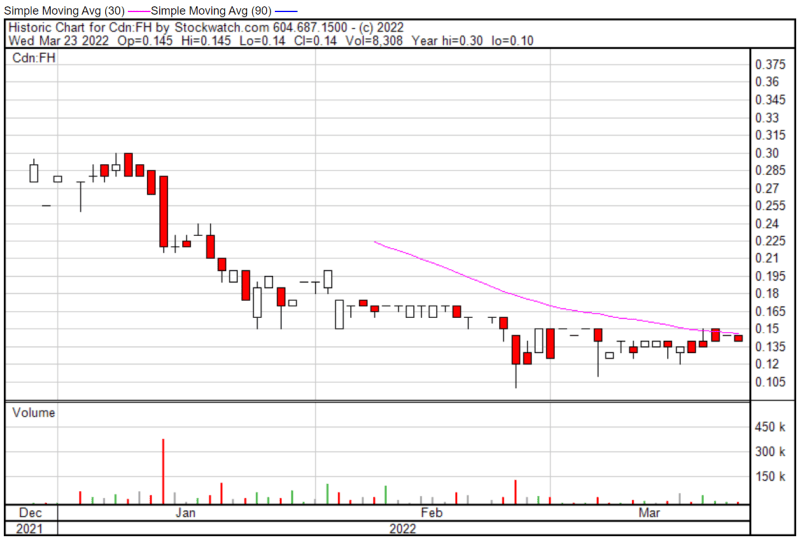
Filament’s share price opened at $0.145 today, compared to a previous close of $0.145. The Company’s shares are down -3.45% and were trading at $0.14 as of 10:20 AM EST.
Full Disclosure: Filament Health Corp. (FH.NE) is a marketing client of Equity Guru.