- $41.189M Market Capitalization
Filament Health Corp. (FH.NE) announced today that it has received approval from Health Canada for a Phase 2 clinical trial utilizing the Company’s standardized natural psilocybin drug candidate PEX010. The trial will evaluate the safety and efficacy of low doses of psilocybin in healthy subjects with persistent depressive disorder.
“We are pleased to support this landmark Canadian trial through the donation of our standardized natural psilocybin…Our team’s involvement in facilitating this trial is an important contribution to the spirit of psychedelic research…
We have greatly enjoyed working with Rotem and his team, and look forward to continuing to closely collaborate on this trial. We are excited to learn about new applications for our drug candidates and to gather additional safety data,” said Filament Chief Executive Officer, Benjamin Lightburn.
Filament Health’s three leading drug candidates, PEX010, PEX020, and PEX030, are proprietary, naturally-extracted mushroom formulations. In particular, the Company’s PEX010 is an oral compound of psilocybin intended to treat Major Depressive Disorder (MDD), which refers to persistent and intense feelings of sadness for extended periods. As a result, MDD can affect many areas of one’s life including mood and behavior as well as various physical functions, including appetite and sleep.
MDD Market Growth

It is worth noting that MDD is one of the most common mental health conditions in the United States (US). According to the National Institute of Mental Health, more than 7.8% of US adults experienced a major depressive episode in 2019. When we consider the fact that the first confirmed case of COVID-19 was reported in the US in 2019, this isn’t too surprising. A growing number of adults with MDD in the US can largely be attributed to increased financial and emotional distress caused by the coronavirus.
With gyms, restaurants, and schools once again closing in Ontario, I am bound to lose my marbles in the near future. However, while COVID-19 may suck, it has had a positive impact on anxiety disorders and depression treatment market growth due to a surge in the sales of products for the management of mental health conditions. In fact, according to Fortune Business Insights, prescriptions for anxiety and depression drugs spiked up 34.1% and 18.6% respectively, during 2020.
In total, the Global Anxiety and Depression Treatment Market was valued at USD$8.50 billion in 2019. With COVID-19 still terrorizing the globe, this market is expected to reach USD$13.01 billion in 2027, expanding at a compound annual growth rate (CAGR) of 2.6% during the 2027 to 2027 period. With this in mind, research has shown that psychedelic medications like psilocybin are capable of treating a wide range of mental health disorders, including MDD and anxiety. For example, a study conducted by Johns Hopkins Medicine revealed that two doses of psilocybin, in combination with supportive psychotherapy, produced rapid and large reductions in depressive symptoms.
Phase 2 Clinical Trial

Referring back to Filament’s latest news, the Phase 2 clinical trial will be led by Rotem Petranker, Director of the Canadian Centre for Psychedelic Science, and Dr. Norman Farb at the University of Toronto. In terms of funding, the trial has received philanthropic funding from the Nikean Foundation. For context, the Nikean Foundation is an organization dedicated to providing its resources to programs that are advancing psychedelic science with the hopes of building better tools for mental health care.
“The Filament Health team has been instrumental in getting this trial up and running…I am excited to work with Filament, whose commitment to professionalism and Open Science has been unwavering. Filament’s product allows us to closely approximate the conditions under which people microdose in the real world, and I expect the results from this study to be very informative,” said Rotem Petranker, Director of the Canadian Centre for Psychedelic Science and the study’s Principal Investigator.
As for the trial itself, the placebo-controlled Phase 2 clinical trial is expected to begin dosing in the first quarter of 2022. The trial has been designed to include 100 healthy subjects experiencing symptoms of persistent depressive disorder. Ultimately, the trial will examine the effects of Filament’s botanical drug candidate PEX010 (1 mg), a newly-developed microdose formulation based on PEX010 (25 mg), which previously received approval from the FDA to enter into a Phase 1 clinical trial at the University of California San Francisco.
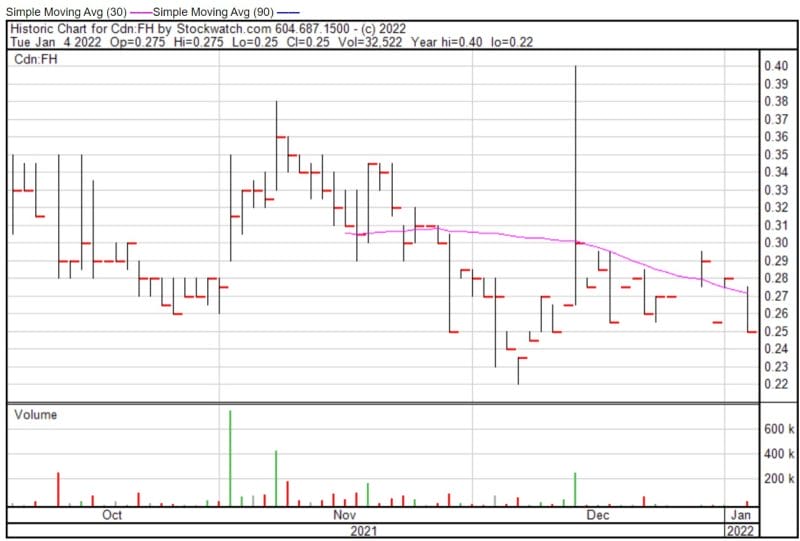
Filament’s share price opened at $0.275, down from a previous close of $0.28. The Company’s shares are down -10.71% and were trading at $0.25 as of 9:46 AM EST.
Full Disclosure: Filament Health is a marketing client of Equity Guru.