Filament Health (FH.NEO) announced today that it has been granted an amendment to its existing Health Canada Dealer’s License, bolstering the Company’s position at the forefront of natural psychedelic research and manufacturing.
“This license amendment significantly increases the scope of our work with natural psychedelics…By studying untapped psychedelics in a scientific setting, we believe we can unlock and standardize their healing power. This is a promising step forward in our mission to get safe, natural psychedelics into the hands of everyone who needs them, as soon as possible,” said Filament’s Director of Research, Ryan Moss.
Who is Filament Health?
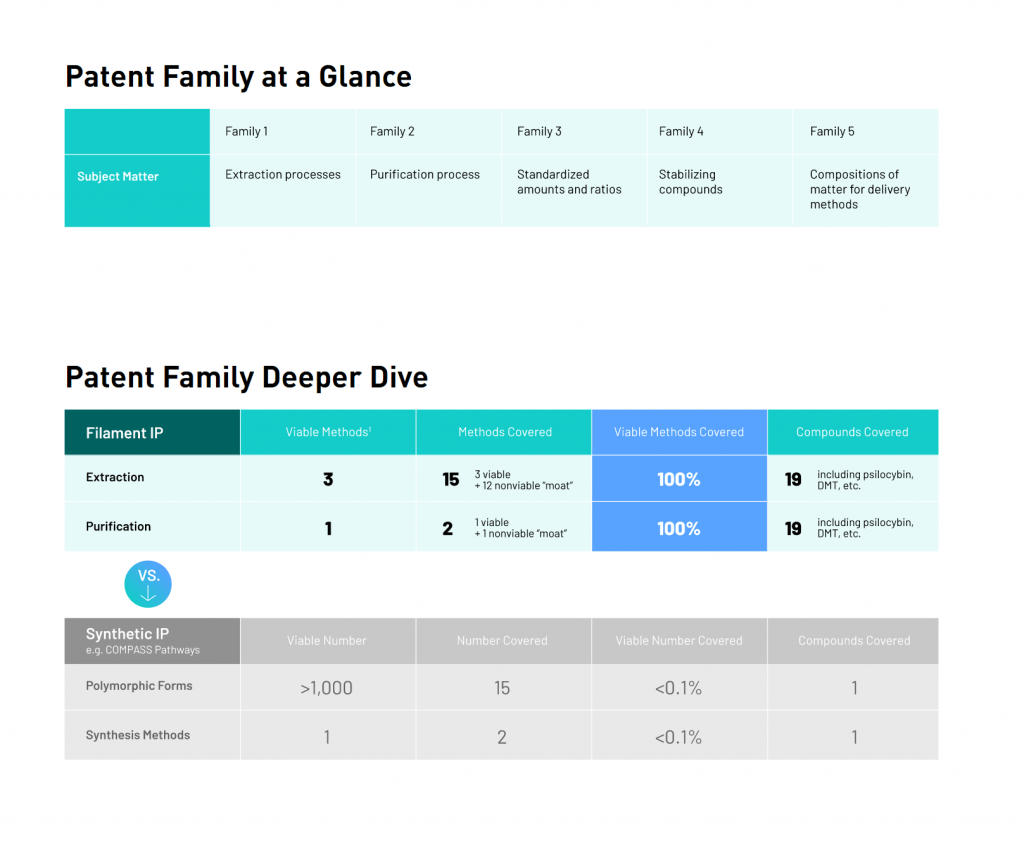
Filament Health is a leading exclusively natural psychedelic drug development company focused on building a powerful platform to support the treatment of mental health conditions, some of which include depression, anxiety and addiction. In North America alone, roughly USD$280 billion is spent on mental health treatment. With this in mind, there are over 130 active clinical trials globally demonstrating or seeking to demonstrate the potential of psychedelics in treating mental health conditions. However, with an innovative IP portfolio, in-house GMP manufacturing capabilities, and a Health Canada psilocybin Dealer’s License, Filament is uniquely positioned to combat the ongoing mental health crisis.
Filament’s GMP facility is one of the first in the world to also have a Health Canada Dealer’s License, which enables the Company to propagate psychedelic plants, conduct genetic research, perform extraction procedures, run in-house trial, and distribute IP and drug candidates. Currently, Filament’s GMP facility manufactures drug candidates naturally extracted from psychoactive mushrooms. However, the recent amendment to the Company’s Health Canada Dealer’s License will allow the possession, production, research, supply, export, import and delivery of all remaining controlled psychedelic substances including DMT, harmaline and mescaline, among others.
“We are encouraged by this development, which we believe strengthens Filament’s position and opens up new revenue sources for us…The benefits of these valuable plants are well-documented; we will be among the first to purposefully explore their pharmaceutical applications,” commented Filament Chief Executive Officer Benjamin Lightburn.
The compounds covered by Filament’s license amendment have a long history of use in traditional medicine and recreational settings. Not convinced? In a study published by the American Chemical Society, 68-86% of the respondents who consumed mescaline in naturalistic settings self-reported improvements for a variety of clinical conditions including depression, anxiety, PTSD, and drug use disorders. Furthermore, in the ancient village of Eleusis, for more than 2000 years there was an annual all-night secret ceremony believed to have involved the ingestion of a hallucinogenic brew known as κψκεον. Aside from sounding like a great time, this ceremony was recognized for invoking profound insight about life. With this in mind, utilizing its unique drug development platform, Filament intends to develop naturally derived, standardized preparations of these compounds, and study their applications for therapeutic use in treating a wide range of health conditions.
Looking forward, Filament will produce its psychedelic extracts in-house at its Vancouver-based GMP-certified facility. Additionally, the Company has also developed compounds which will be studied during the first ever FDA-approved natural-psilocybin clinical trails. Filament has received approval from the IRB (Institutional Review Board) to proceed with its PEX020 and PEX030 vs. PEX010 clinical trial which is expected to begin in Q3 2021.
Full Disclosure: Filament is a marketing client of Equity Guru.