Medexus Pharmaceuticals (MDP.TO) today announced preliminary estimates of the company’s revenue results for the quarter ended December 31st 2022 (fiscal Q3 2023). After Q2 2023 being Medexus’ strongest quarter in company history, Medexus’ core business continues to grow with another record breaking quarter expected.
Medexus expects total revenue for fiscal Q3 2023 to be between $28.5 million and $29.0 million (all amounts in this article are in US dollars). This would be another record quarter for the company and a year-over-year increase of close to 34% and a quarter-over-quarter increase of at least 3%.
An increase in net sales across Medexus’ portfolio is one of the primary drivers for improved revenue:
- IXINITY: Positive trend in sales, reflecting new patient conversions on top of a stable, existing base of patients.
- Rasuvo: Continued strong performance and maintenance of the product’s leading position in the moderately-growing US branded methotrexate market with a limited sales force allocation.
- Rupall: Continued strong demand exhibiting typical seasonality as compared to fiscal Q2 2023, reflecting successful execution of the company’s sales and marketing initiatives and sustaining the product’s strong performance over the five years since launch.
- Gleolan: Continued positive trend in US sales, positioning Medexus to successfully execute its post-transition commercial plan including new sales and marketing initiatives.
The company also provided a business update to shareholders pertaining to Treosulfan and current business strategy.
Following up on Medexus’ last update on Treosulfan in November 2022, Medac, the party responsible for regulatory matters for Treosulfan, has continued to engage with the US Food and Drug Administration (FDA) when it comes to Medac’s resubmission of its new drug application for Treosulfan. After the FDA’s second notice of incomplete response delivered to Medac in September 2022, Medac is primarily focused on establishing the most appropriate approach to addressing the remaining items noted.
Medexus expects it would take Medac up to a year or more to collect and submit the information requested by the FDA. The FDA would then evaluate the completeness of the available information submitted and Medac’s response and, if considered to be complete, then proceed to review Medac’s NDA resubmission.
Medexus believes that treosulfan would make a substantial difference for US patients and therefore continues to urge Medac to take the steps necessary to respond to the FDA’s requests in a timely and complete fashion and fulfill Medac’s obligations under the terms of the Treosulfan license agreement to pursue all commercially viable paths to completing the NDA resubmission.
In the near term, Medexus continues to focus on commercializing its current product portfolio and seeking out additional product opportunities while growing its core business and delivering strong financial results.
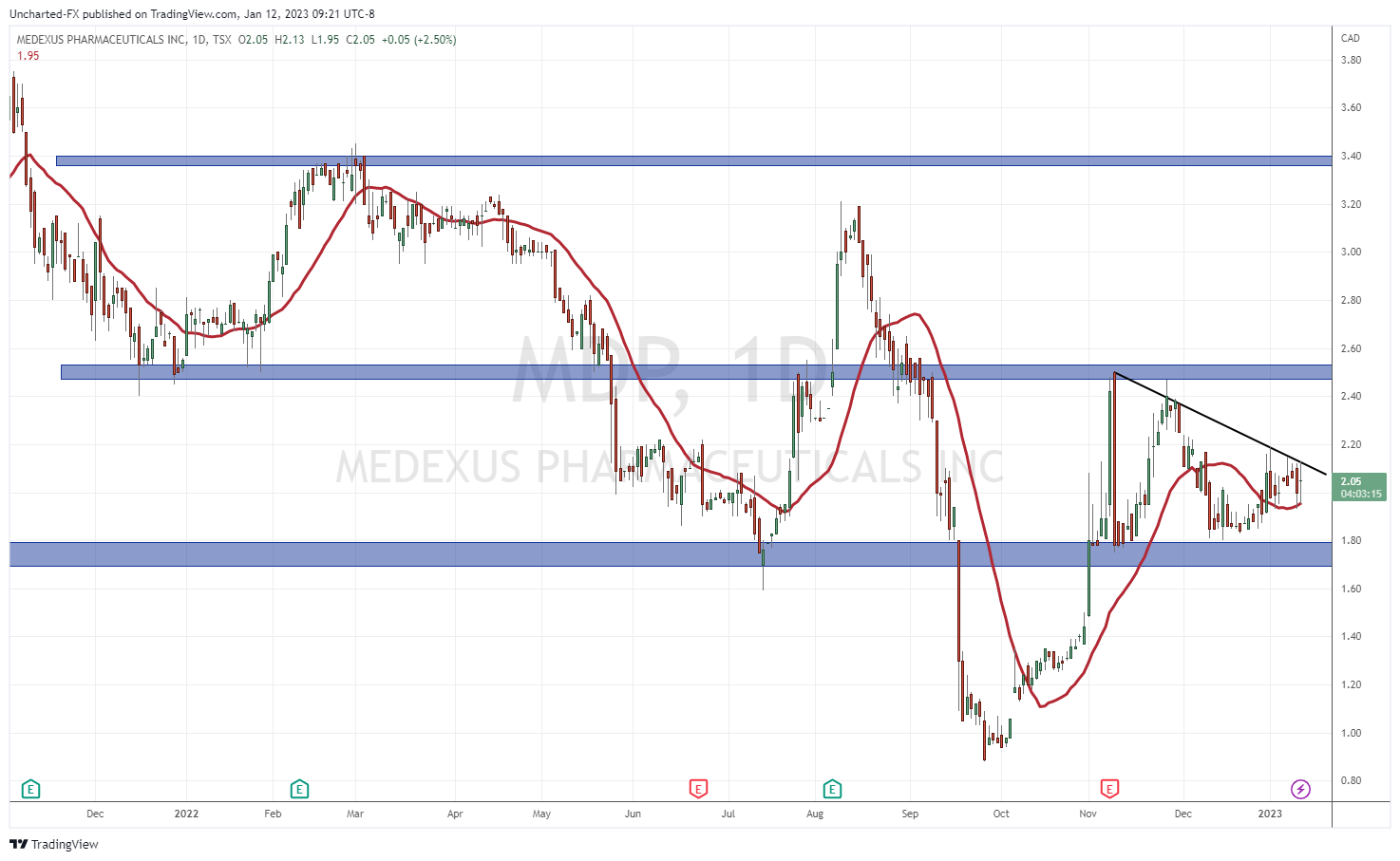
At time of writing, the stock is up 2.50% with over 58,000 shares traded on the preliminary results news.
When it comes to the technicals, the support and resistance levels I have highlighted in previous Medexus articles are still in play.
Support comes in at $1.80, and resistance comes in at $2.50. The breakout above the resistance level sets us up for a major run. Perhaps the official earnings news release will be the catalyst.
I want to draw traders and investors attention to the downtrend line I have drawn out. A line the stock is testing today, and has tested and respected for the past few trading days. If the stock can confirm a close above this trendline, that could initiate an initial rally which would take us to the $2.50 zone. Nonetheless, it would be an important breakout since the stock has adhered and remained below this trendline multiple times.