Medexus Pharmaceuticals (MDP.T), a Canadian-based specialty pharma company focused on acquiring and distributing innovative rate disease treatment solutions, announced its financial results for Q1 2023.
The company reported a total revenue of $23.0 million USD for the quarter, a 33% increase versus Q1 2022’s $17.3 million and a 13% increase versus Q4 2022.
The quarter was the strongest fiscal Q1 in Medexus’ history thanks to the company’s increase in net sales of IXINITY and a recognition of a portion of revenue from Gleolan sales in the US.
Medexus also recorded an adjusted EBITDA of $1.9 million in the quarter compared to $(4.9) million in Q1 2022 and $1.1 in Q4 2022. The company was able to achieve this increase while continuing to maintain investments necessary for a commercial launch of treosulfan in the US.
Operation profit was $0.0 million in Q1 2023 compared to an operating loss of $7.2 million in Q1 2022.
The pharma firm produced a net loss of $1.4 million versus a net loss of $6.6 million in Q1 2022, a 78% improvement.
Medexus reported cash and cash equivalents of $7.3 million (with $8.7 million of total available liquidity) at the end of Q1 2023.
Ken d’Entremont, Medexus CEO, commented, “We are proud to announce the strongest fiscal Q1 in our company’s history driven by a strong base business and complemented by initial revenues generated through our license agreement for Gleolan in the U.S. We anticipate completing our transition to full commercial responsibility for Gleolan within the current quarter and will begin to report Gleolan net sales in our revenues starting at that time. So far Gleolan sales have performed to our expectations, and we are excited about Gleolan’s contribution to growing our revenues over the coming quarters.”
Operationally speaking, product revenue for IXINITY increased over the quarter as pharmacies and wholesale customers have returned to normal buying patterns.
Q1 2023 also saw continued strong growth in Rupall sales with unit demand hitting a 22% increase for the 12 months ending June 30, 2022.
The process for transition of commercialization responsibility for Gleolan is well under way and is expected to complete in the current quarter, resulting in Medexus having full responsibility for commercializing Gleolan in the US.
Treosulfan’s NDA resubmission was announced in July 2022. The resubmission included updates to data files and supporting information in response to the FDA’s information request received back in May. Also, Medexus and its strategic partner, medac signed an amendment to their February 2021 license agreement for treosulfan, which extends the payment dates for regulatory milestones triggered by an FDA approval to October 2023. This means Medexus can launch and begin commercialization well before these license payments must be paid.
In other news, Stifel Financial Corp, an American multinational independent investment bank, released its analysis on Medexus back at the end of July noting, “We see MDP as a specialty pharma company with solid offerings of IXINITY for hemophilia B, Rasuvo and Metoject in rheumatoid arthritis, a fast growing allergy medicine, Rupall, along with the business being at a point of inflection for operating leverage with the infrastructure in-place to support much greater sales. As scale is achieved, profit is expected to ramp, leading to a re-rating in valuation and a much higher share price.”
Stifel gave Medexus a buy rating and a target price of $14.25 CAD.
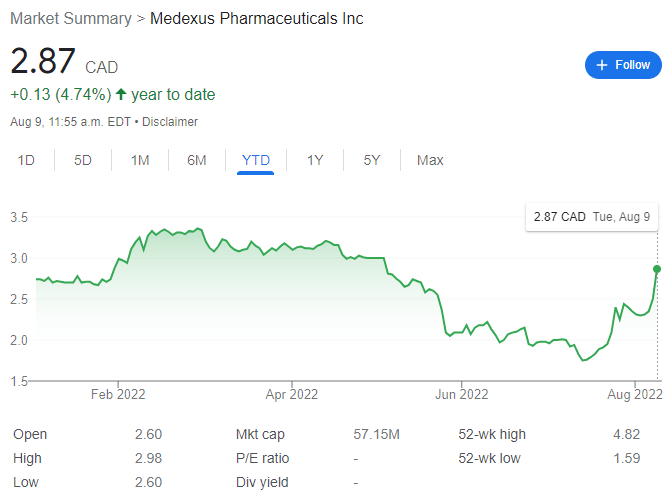
Medexus currently trades at $2.87 per share for a market cap of $57.15 million.
–Gaalen Engen