Closing the Diabetic Loop

Before we get into this week’s sector roundup, let’s talk about diabetes. I am a sucker for sweets, so much so that I was diagnosed as prediabetic some moons ago. Thankfully, I appear to have narrowly avoided the sugar-coated grasp of diabetes. Unfortunately, others aren’t so lucky. According to a study published in Diabetes Research and Clinical Practice, global diabetes prevalence in 2019 was 9.3%.
By 2045, this number is expected to grow to 10.9% or 700 million people, representing an increase of 51% since 2019. To make matters worse, approximately one in two people living with diabetes are unaware that they have the disease. If that wasn’t enough, Millennials are disproportionately affected by behavioral health conditions when compared to the national population of the United States (US).
Some of these health conditions include major depression, hyperactivity, and type II diabetes, among many others. In particular, the prevalence of type II diabetes amongst US Millennials between 2014 and 2017 has increased by 22%. However, times are changing and treatments for diabetes are reaching new heights. For example, Tandem Diabetes Care Inc. (TNDM.Q) is putting diabetes treatment in the palm of our hands.
Specifically, Tandem hopes to enable people with diabetes (PWDs) to control their medical devices through a mobile smartphone app. In doing so, PWDs will have access to various functionalities, including remote insulin dosing. Before you get too excited, Tandem has not yet received approval from the Food and Drug Administration (FDA) for use in diabetic devices.
Though, there is hope yet! On February 16, 2022, Tandem announced that it had received FDA clearance for its mobile app for iOS and Android smartphones. With this in mind, the company expects to launch its application as early as Summer 2022. Aside from Tandem, companies like Medtronic are making advancements toward creating a closed-loop system for insulin delivery.
This is commonly referred to as an artificial pancreas. In case you didn’t pay attention in biology class like me, the pancreas is responsible for producing insulin, a hormone that allows the body to use sugar from carbohydrates for energy or store it for use in the future. However, for PWDs, the pancreas does not produce insulin. As a result, PWDs are required to administer insulin themselves.
With this in mind, an artificial pancreas automatically monitors a PWD’s blood glucose level, calculates the amount of insulin needed, and delivers it. Referring back to Medtronic, a combination of the company’s MiniMed 780G and Guardian 4 sensor has demonstrated the ability to keep users within their ideal glucose range for an average of 73% of the day.
With promising results under its belt, Medtronic has now chartered a course toward a completed closed-loop system. To start, the company plans to eliminate the need for PWDs to analyze every meal they eat to adjust their insulin doses. Medtronic will utilize an algorithm embedded in a smartwatch to detect when a PWD is eating and for how long. In doing so, the algorithm will determine how much insulin is needed.
It’s not all good news though. In February 2021, Medtronic received a warning from the FDA following the inspection of the company’s Northridge, California, facility. Keep in mind that this facility represents Medtronic’s diabetes segment. The FDA highlighted shortfalls in the segment’s risk assessment, corrective and preventive action, complaint handling, device recalls, and adverse event reporting.
Having suffered a blow from the FDA, Medtronic has indicated that approval timing for the company’s MiniMed 780G insulin pump and Guardian 4 sensor is now uncertain. Nonetheless, Medtronic expects to launch its products sometime in the coming quarters. The company has also invested in numerous diabetes programs associated with next-generation insulin pumps and patch-pump technologies.
Lexaria Bioscience Corp.
- $13.747M Market Capitalization
Lexaria Bioscience Corp. (LEXX.Q) is a biotechnology company developing DehydraTECH™, the Company’s proprietary drug delivery technology. In addition to enhancing the speed and efficiency of orally-delivered drugs, DehydraTECH has demonstrated effectiveness in improving delivery through human skin for the potential development of topically-administered products such as patches.
Through various animal studies evaluating the quantity of drug delivery across the blood-brain-barrier (BBB) utilizing DehydraTECH technology, data suggests a gain of as much as 1,900%. With this in mind, Lexaria’s DehydraTECH has the potential to improve the delivery of certain central nervous system (CNS) targeted drugs against Alzheimer’s Disease, Parkinson’s Disease, and other CNS diseases.
Latest News
On June 8, 2022, Lexaria announced that it has awarded AnodGen Bioceuticals a five-year, non-exclusive DehydraTECH license. With this license, AnodGen can manufacture and distribute DehydraTECH processed cannabinoid active pharmaceutical ingredient (API) powders within Europe, including the United Kingdom (UK), Australia, and New Zealand.
“This partnership with Lexaria will help bring research-based APIs and technology to companies and people with superior bioavailability and enhanced absorption. This alliance will bring about the change we will yet see in pharmacokinetics and formulation,” said Dr. Jeffrey Pruski, Anodgen’s Chief Medical Officer and Founding Member.
Additionally, AnodGen has the right to manufacture and sell these API powders to third-party companies for their own products. According to the terms of the agreement, AnodGen will pay royalty fees to Lexaria for all API powders sold utilizing the Company’s DehydraTECH. However, consumer products purchased without a physician or medical professional consultation are prohibited.
Why AnodGen? Well, AnonGen is a contract manufacturing organization business. The company intends to distribute APIs within the pharmaceutical industry. More importantly, AnonGen expects to have its new facility in Ireland fully operational sometime this year, which will substantially augment the company’s capabilities in Europe. As such, Lexaria has recognized the long-term potential of AnodGen.
It should be noted that AnodGen isn’t the only company that Lexaria has issued a license to. On June 2, 2022, Lexaria announced a significant exclusive commercial licensing agreement in Japan with Premier Wellness Science Co. Ltd. In addition to paying royalties to Lexaria, Premier will make negotiated minimum quarterly payments to the Company beginning on September 1, 2022.
“Lexaria is delighted to be working with Premier Wellness Science which we expect will dominate the newly opening Japanese market for CBD-based products…We could not have found a better partner with which to introduce DehydraTECH-enabled products to the Japanese markets,” said Chris Bunka, CEO of Lexaria.
During the first five years of this agreement, Premier will pay a total of USD$4,527,500 to Lexaria. Even if Premier achieves its worst-case projected penetration into Japan’s CBD market, Lexaria is still expected to receive annual payments of greater than $5 million by the fifth year of the contract. With this in mind, Lexaria’s licensing agreement with Premier represents a promising revenue stream.
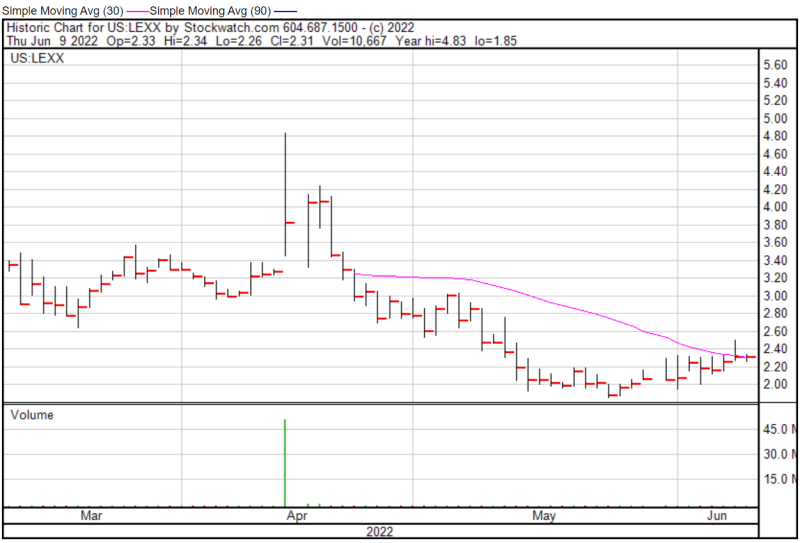
Lexaria’s share price opened at $2.33 on June 9, 2022, up from a previous close of $2.31. The Company’s shares were trading at $2.31 as of 11:29 AM EST.
Tetra Bio-Pharma Inc.

- $25.378M Market Capitalization
Tetra Bio-Pharma (TBP.T) is a biopharmaceutical company focused on cannabinoid-derived drug discovery and development. The Company has an FDA and Health Canada cleared clinical program as well as an extensive pipeline of drug products for a range of medical conditions, including pain, inflammation, and oncology, among others.
In total, Tetra’s cannabinoid-derived medicines target medication indications representing addressable markets of more than $20 billion, supported by the urgent need for non-opioid alternatives. In particular, Tetra has developed REDUVO™, a soft gel capsule used to treat chemotherapy-induced nausea and vomiting (CINV) as well as weight loss and severe nausea in people living with HIV.
Latest News
On June 6, 2022, Tetr announced the launch of its wholly-owned subsidiary, Tetra Bio-Pharma Australia Pty Ltd. The Company’s latest Australia-based research company will focus on the execution of clinical trials in Australia. This represents Tetra’s second foreign subsidiary and is in line with the Company’s global expansion strategy for QIXLEEF and other drug candidates.
“We look forward to working with our strategic partners and building value for our current and future investors. These are very exciting times for us as we continue to drive scientific excellence and deliver on the promise of cannabinoid-derived transformative medicines to improve patient health and quality of life,” said Tetra’s CEO, Dr. Guy Chamberland.
To provide some background, QIXLEEF is Tetra’s botanical inhaled drug candidate characterized by a fixed ratio of THC and CBD. QIXLEEF is intended for pain treatment and has been evaluated extensively via the REBORN and PLENTITUTE trials. For more information regarding QIXLEEF, check out this article!
Speaking of clinical trials, on May 5, 2022, Tetra announced a research and development agreement with Cannvalate Pty Ltd. With this in mind, Tetra’s latest subsidiary is a product of the Company’s latest partnership with Cannvalate. Through Cannvalate, Tetra is now able to initiate clinical trials of its drug candidates in Australia. One of the most apparent upsides to this partnership is a 43.5% tax credit on all money spent on clinical trials in Australia.
According to the terms of the agreement, Cannvalate has agreed to acquire common shares of Tetra on a private placement basis, via seven distinct tranches, for total proceeds of CAD$7,500,000. The first tranche, valued at CAD$500,000, was completed following the signing of the agreement. However, subsequent tranches will be triggered by the completion of various operational events related to clinical trials.
In Tetra’s latest press release, the Company also provided a regulatory update for REDUVO. Tetra has stated that it has completed the annual license review for its Health Canada Establishment License (DEL) and meets the regulatory requirements of the Food and Drug Regulations. To summarize, this allows Tetra to continue distributing pharmaceuticals like REDUVO in Canada.
Currently, Tetra is in discussion with Health Canada regarding the regulatory approval of REDUVO. With this in mind, the Company has indicated that it is ready to launch manufacturing activities as soon as it receives regulatory approval from Health Canada. Keep in mind that the global CINV treatment market is expected to reach USD$5.7 billion by 2031, representing a market opportunity for REDUVO.
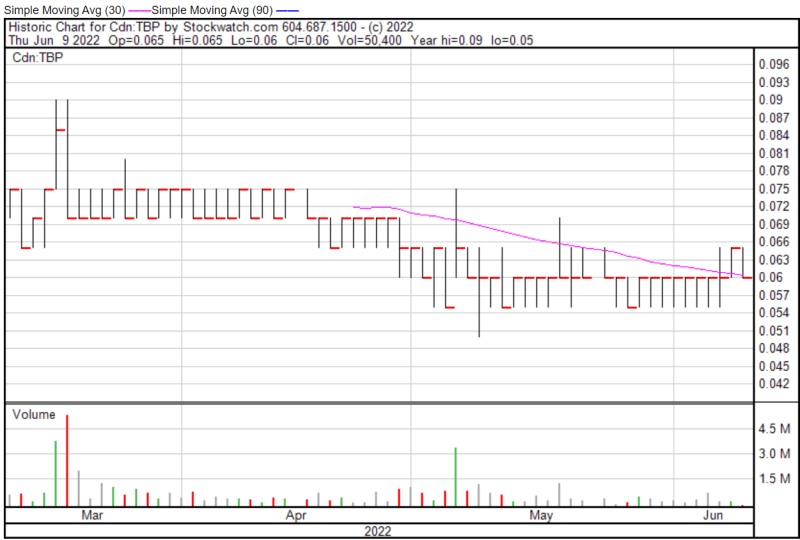
Tetra’s share price opened at $0.065 on June 9, 2022, compared to a previous close of $0.065. The Company’s shares were down -7.69% and were trading at $0.06 as of 11:46 AM EST.
BioVaxys Technology Corp.

- $11.726M Market Capitalization
BioVaxys Technology Corp. (BIOV.C) is a clinical-stage biopharmaceutical company developing anti-viral and anti-cancer vaccine platforms. Currently, the Company is evaluating a potential SARS-CoV-2 vaccine based on its haptenized viral protein technology. BioVaxys is also advancing a compassionate use trial in the EU to evaluate its haptenized cell vaccine for late-stage ovarian cancer.
In addition to BioVaxys’ cell vaccines for ovarian cancer, the Company is also exploring other ways it can leverage its technology platform in the field of adoptive immunotherapy. For context, adoptive immunotherapy refers to the collection of T-cells from a patient that are then grown in a lab. This increases the number of T-cells that can be used to kill cancer cells.
Latest News
On June 8, 2022, BioVaxys announced that its France-based bioproduction partner, BioElpida, has completed the creation of multiple ovarian OVCAR-3 cell banks. This represents the next step in the Company’s GMP manufacturing process development for BVX-0918, BioVaxy’s lead haptenized tumor cell vaccine for the treatment of platinum-resistant ovarian cancer.
“Completion of OVCAR-3 cell banks is another step towards our ability to produce GMP yields of BVX-0918 and brings us closer to our Phase I study in the EU planned for later this year,” commented BioVaxys President and Chief Operating Officer, Kenneth Kovan.
BVX-0918 utilizes a patient’s own cancer cells, which are then treated using haptenization and injected back into them. This elicits an immune response, whereby cancer cells are more visibly exposed and vulnerable to T-cells. Ultimately, the primary objective for BVX-0918 is to obtain Good Manufacturing Practice (GMP) validity. So, why is this important?
Aside from ensuring that a product is consistently produced and controlled, a medical GMP certification can boost pharmaceutical export opportunities for a company. In fact, most countries will only accept the import and sale of medicines that are GMP certified. With this in mind, a GMP certification will allow BioVaxys to target the global ovarian cancer drug market, which is expected to reach USD$6.58 billion by 2026.

Additionally, on June 3, 2022, BioVaxys announced that its research collaborator, Ohio State University, has completed preparations of the surrogate virus neutralization assays for the SARS-CoV-2 variants, as well as the Pangolin-CoV-GD1 and Bat-CoV-RaTG13 subvariants. With this in mind, the Company now intends to begin immunization of test animals with BVX-1021.
“The repeated emergence of SARS-CoV-2 variants and the potential for new coronaviruses increases the urgency for a universal vaccine. Research suggests that a pan-sarbecovirus vaccine could potentially prevent additional emergent variants and help end the Covid-19 pandemic,” said Kenneth Kovan.
BioVaxys first began collaborating with Ohio State University following its announcement on December 7, 2021. According to the Company, this collaboration is intended to further develop BioVaxys’ viral antigen platform to create a broadly reactive pan-sarbecovirus vaccine. For context, sarbecoviruses include the SARS2 variants Delta and Omicron.
This collaboration is specifically evaluating a combination of BioVaxys’ BVX-0320 and BVX-1021 in a guinea pig model. BVX-0320 is BioVaxys’ lead IND-stage vaccine candidate for SARS-CoV-2 and has demonstrated a 96.4% antibody response during in vivo studies. If successful, BioVaxys will have developed a vaccine capable of inducing immunity across all or most sarbecoviruses.
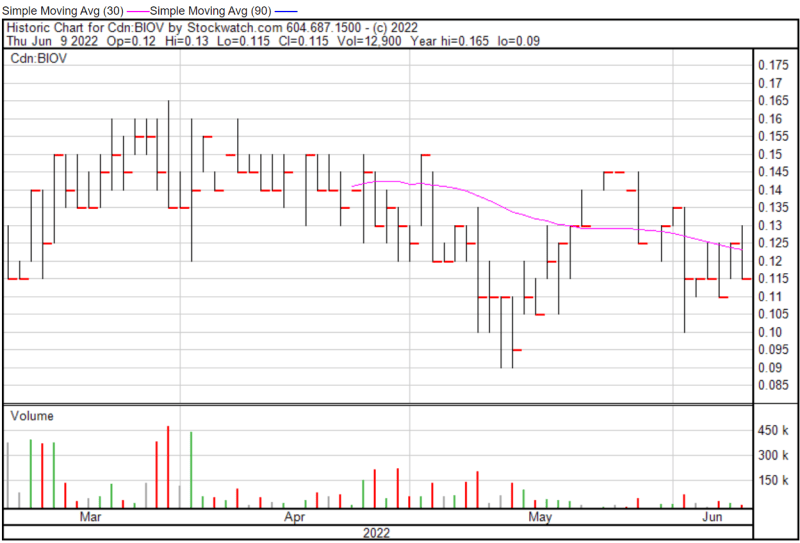
BioVaxys’ share price opened at $0.12 on June 9, 2022, down from a previous close of $0.125. The Company’s shares were down -4.00% and were trading at $0.12 as of 12:40 PM EST.