Filament Health (FH.NE) announced today that it has received approval from Health Canada for a Phase 2 clinical trial using PEX010, the Company’s botanical psilocybin drug candidate. Cybin Therapeutics has licensed the Company’s PEX010 from Filament for use in the trial, which is expected to begin in Q3 2022. The trial will include individuals with major depressive disorder (MDD) who are undergoing selective serotonin reuptake inhibitor (SSRI) therapy, as well as those who are SSRI-naive.
“This agreement with Cybin Therapeutics demonstrates Filament’s ability to license our technology and facilitate our partners’ trials…Our mission is to support the treatment of mental health conditions through the discovery and delivery of exclusively-natural psychedelic medicines, and we are proud to aid CT’s efforts. We view this as the first of many agreements in a long partnership,” said Filament Chief Executive Officer, Benjamin Lightburn.
Not to be confused with Cybin Inc. (CYBN.NE), Cybin Therapeutics is a private therapeutic bioscience company committed to developing psilocybin-assisted therapeutic protocols to treat mild mood disorders and addictions. Cybin’s latest licensing agreement with Filament will enable Cybin to use PEX010 in its upcoming clinical trials addressing depression and alcohol use disorder.
With this in mind, depression is one of the most common illnesses worldwide, with an estimated 3.8% of the population affected, including 5% among adults and 5.7% among adults older than 60 years. To put things into perspective, this suggests that approximately 280 million people in the world have depression, according to the World Health Organization (WHO). Unfortunately, over 700,000 people die to suicide, the fourth leading cause of death in 15 to 29-year-olds, every year.
Currently, there are multiple treatments for depression including behavioral activation, cognitive behavioral therapy (CBT), and antidepressant medications, to name just a few. Despite this, there are several barriers to effective treatment for depression such as lack of resources, shortage of trained health care providers, and social stigma associated with mental disorders like depression and alcohol use disorder (AUD).
Speaking of which, an estimated 14.5 million people ages 12 and older had AUD according to the 2019 National Survey on Drug Use and Health (NSDUH). Similar to depression, common treatments for AUD include medicines and behavioral therapies, or both. With this in mind, research has shown that psychedelic medication like psilocybin and MDMA are capable of opening new pathways in the brain that, in conjunction with professional therapy, can treat a wide range of mental health disorders.
For example, a study conducted by Johns Hopkins Medicine revealed that two doses of psilocybin, in combination with supportive psychotherapy, produced rapid and large reductions in depressive symptoms. Referring back to Filament’s latest news, Cybin filed a Clinical Trial Application (CTA) to Health Canada for its first clinical trial in 2021 and anticipates its depression study to begin in Q3 2022.

Both trials will be led by Dr. Reg Peters and Dave Philips. Dr. Peters has been involved in advocacy for the compassionate use of psilocybin for palliative patients, including as a medical advisor to TheraPsil, a non-profit coalition dedicated to helping Canadians in medical need access legal, psilocybin-assisted psychotherapy. Mr. Dave Phillips, Training Advisor to TheraPsil and a highly experienced psilocybin-assisted therapist, will lead the therapy component of the trials along with an experienced clinical team at Cybin Therapeutics’ purpose-built clinic.
“We are excited to advance our mission of creating psilocybin-based therapies for a number of treatment areas. Filament’s unique, all-natural drug candidates combined with our experienced team, we believe, create an ideal environment for creating research-based protocols, formulations, and outcomes for healing,” commented Josh Taylor, Cybin Therapeutics Founder.
Additionally, due to strong commercial demand for the Company’s unique proprietary drug candidates, Filament announced that it has engaged business development professionals to meet inbound requests and begin commercial outreach. Andry Tjahyana has joined Filament as Vice President, Business Development, bringing more than 25 years of relevant experience. In addition to Mr. Tjahyana, Filament has engaged Thomas Ryan, Rob Grundy, and Christine Verstraate from NavigatorBIO, who bring a combined 65 years of pharmaceutical industry sales experience.
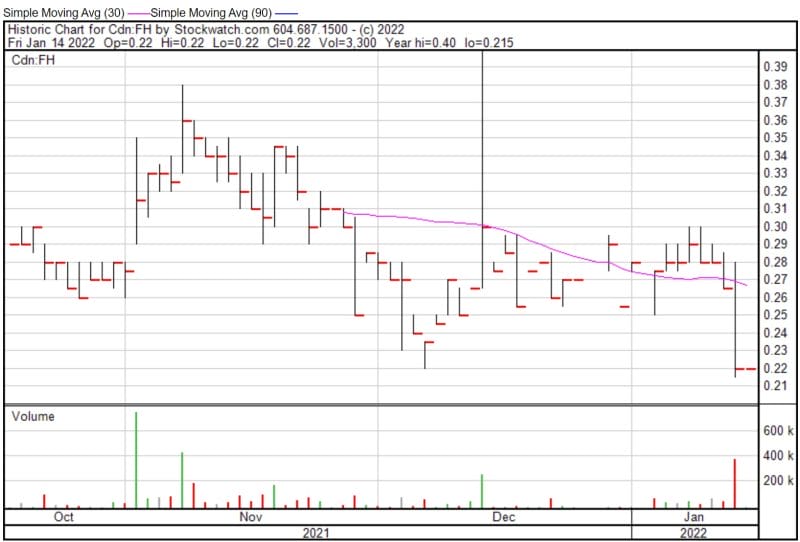
Filament’s share price opened at $0.19, up from a previous close of $0.175. The Company’s shares are up 8.57% and were trading at $0.19 as of 9:44 AM EST.
Full Disclosure: Filament Health (FH.NE) is a marketing client of Equity Guru.