We’re living in interesting times and I mean that in the Chinese curse kind of way. We can’t seem to stop to catch our breath when it comes to this pandemic, and the news sources we trust to keep us informed can’t seem to get the story straight. The result is more isolation, frustration, and anger. We can see the effects to our mental health all over social media.
And it’s not good for us. Any of it.
According to a survey conducted by the World Health Organization (WHO) from June to August 2020 over the 130 countries in the WHO’s six regions, we saw widespread disruption of many kinds of mental health services:
- Over 60% reported disruptions to mental health services for vulnerable people, including children and adolescents (72%), older adults (70%), and women requiring antenatal or postnatal services (61%).
- 67% saw disruptions to counselling and psychotherapy; 65% to critical harm reduction services; and 45% to opioid agonist maintenance treatment for opioid dependence.
- More than a third (35%) reported disruptions to emergency interventions, including those for people experiencing prolonged seizures; severe substance use withdrawal syndromes; and delirium, often a sign of a serious underlying medical condition.
- 30% reported disruptions to access for medications for mental, neurological and substance use disorders.
- Around three-quarters reported at least partial disruptions to school and workplace mental health services (78% and 75% respectively).
The COVID-19 virus and its variants aren’t exactly the Spanish Flu in terms of its death rate, but just because it won’t leave scores dead in its wake doesn’t mean it won’t cause widespread damage. The effects of isolation, and of social media’s constant state of aggravation, and the divisions and gulfs between people over issues raised by the pandemic are exacerbating conditions of the unseen, mostly ignored, mental health crisis going on around the world.
On that note, let’s discuss major depressive disorder.
It affects psychosocial functioning and reduces quality of life. It appears to be caused by genetic and environmental factors, and its diagnosis and management are hard because of the fact that it seems to manifest in different ways for different people, and doesn’t respond well to treatment. At least not traditional treatments, so it’s clear we need to start looking elsewhere to find solutions to this unorthodox problem.
That’s where Kelowna-based MYND Life Sciences (MYND.C) comes in.
MYND Life sciences was founded by Dr. Wilfred Jefferies and Dr. Lyle Oberg with the bring some ease to the world of mental health. Doctor Jefferies is a neuroimmunologist with over 60 patents and 100 publications in some big name medical journals, including Nature and The Lancet. What they’re working on right now is to find the link between depression and neuroinflammation, and develop a drug or series of drugs using compounds found in psilocybin to treat it.
MYND approaches their business from three different directions: diagnosis, treatment and protection, and possesses a revenue model based on developing and selling biomarker detection kits for deriving test results from bodily fluids to make it easier and cheaper than what’s presently out there for biomarker testing.
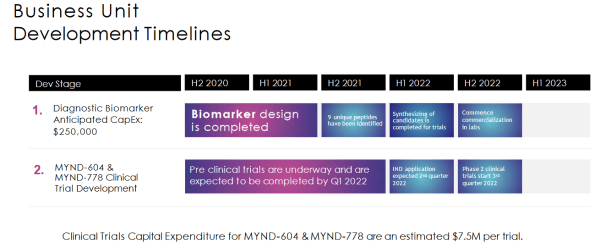
The company has two drug candidates doing the rounds: MYND-604 and MYND-778, which are both clinicals with Investigation New Drug applications expected in Q2, 2022, and Phase II clinicals expected to begin in Q3, 2022.
The Michael Smith Laboratories
The company is presently working with Michael Smith Laboratories at the University of British Columbia. There they perform research in order to develop their understanding of the complex nature of how selected psychiatric and neurological disorders develop. The evidence they’ve gathered thus far suggests a common linkage of several neurological and psychiatric disorders is in neuroinflammation, or inflammation of the brain. Understanding the mechanisms or how this happens will help:
- to identify novel phytochemicals and their analogs with higher therapeutic potential and fewer adverse effects, when compared to existing treatment approaches;
- to create a biomarker-panel for management of (neuro)psychiatric conditions;
- to develop the future partnership with large pharmaceutical companies.
This research is conducted under a part J exemption granted by Health Canada to conduct R&D on restricted and controlled substances.
Alzheimers and better health
The company recently closed a transaction with Cava Healthcare for their intellectual property rights. The specific acquisition is involves the use of psychedelics to treat Alzheimers disease and other types of dementia.
“Dementia, including Alzheimer’s disease, touch almost all families in some way, usually with very dramatic and emotionally painful consequences. As we continue to draw the enthusiasm of investors, we are able take tangible steps towards moving beyond preclinical and into clinical work with our research team,” said Dr. Lyle Oberg, MD, chief executive officer of Mynd.
Alzheimer’s and other types of dementia.
The World Health Organization puts the cost at between 44 million and 50 million people who suffer worldwide from the disease. The estimated financial cost is remarkably higher—as the estimated cost in the United States alone was $305 billion in 2020, according to the Alzheimer’s Association, and is expected to make the leap to $1.1 trillion by 2050. The number of people living with Alzheimers is expected to triple as well by 205 until significant improvements in medical care are made.
This acquisition is a big deal for Mynd because it builds on the company’s experience in the psilocybin-assisted psychotherapy sector, including methodologies or treatment-resistant depression.
The Numbers
Under a ten million dollar market cap so that’s generally a plus—especially when you consider the relative prices of their competitors:
- Mind Medicine (MMED.E) $690,205,000
- Cybin (CYBN.E) $208,172,000
- Field Trip (TRIP.C) $148,329,000
- Numinus (NUMI.T) $122,269,000
- Mydecine Innovations (MYCO.E) $43,053,000
This company at present in the research and development point in their growth cycle—which means no products to put on shelves—and there are a lot of questions unanswered about their future. After all, they have only been public since May.
Their shareholder equity is in the positives, but is honestly nothing to write home about. Their assets and liabilities are close—which again, is not particularly surprising given how nascent this industry (and this company) is. But if you’ll look at the right hand column of this chart, you’ll see it contains no values. Again, public since May. That does represent some growth and the company is trending in the right direction.
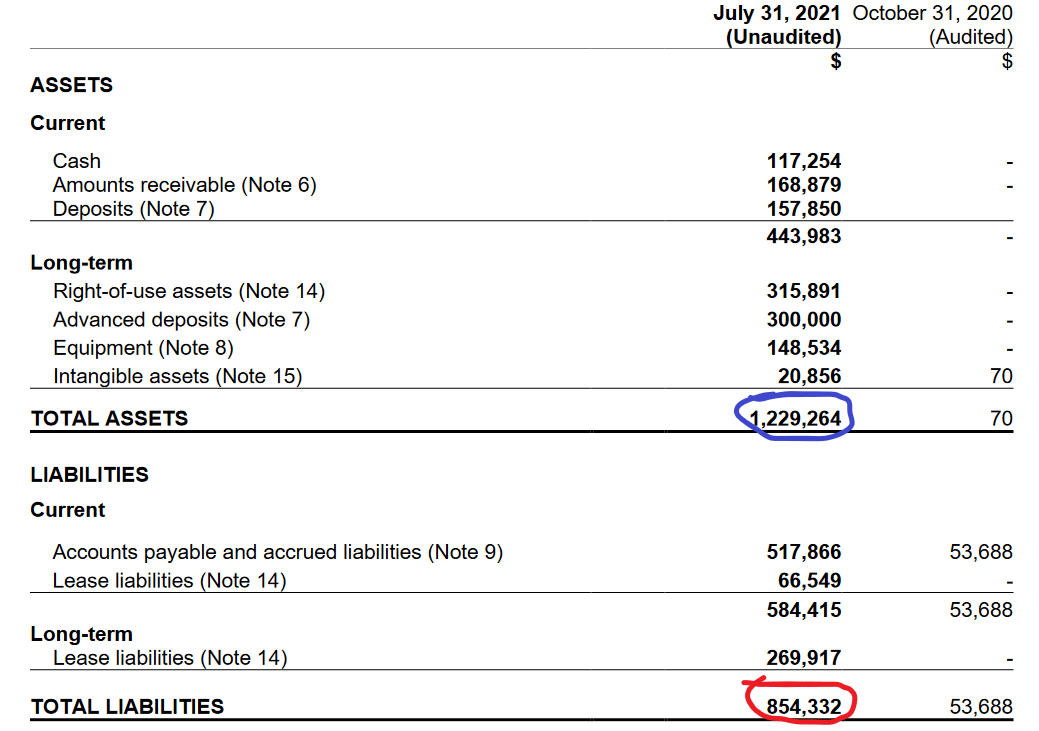
The low six-figure cash position isn’t what anyone would call great, and this company seems to stay alive in most cases due to the largess of scientific grant givers, like the Canadian government.
They pulled in some cash ($45,600) from the National Research Council of Canada Industrial Research Assistance Program for their R&D program that’ll run until March, 2022. The problem with government grants is that a new anti-science Conservative government in full pursuit of guns, Jesus, oil and NO DRUGS could strip away this renewing cash source.
Thankfully this ideological position is in decline among conservatives in Canada, but if there’s anything we can discern from politics since 2015, it’s that politics is unpredictable. It’s improbable, but not impossible that the tide could turn.
But this company has proven both resilient and resourceful in terms of getting cash in their pockets:
They’ve gotten their biomarker test technology into a clinical trial in Australia’s Monash University, and will be seeking partial funds from a $15 million fund offered by the Australian government.
The study is called: “Evaluating the efficacy of psilocybin-assisted psychotherapy in treatment resistant depression” and Mynd Diagnostics will play an integral role in the evaluate component.
If it’s successful, it will mean transition to a multisite phase 3 adaptive trial to take the formulation through to get the regulatory nod from the Therapeutic Goods Association, which is the Australian counterpart to Health Canada.
It’s early yet, and yes there’s an emergent need and subsequent demand for the type of product MYND is working on. But if you’re still looking around for a company with a stable growth trajectory in the future, this might be one to look at. As always, though, if you’re into more of a technical perspective, then feel free to check out our resident chart wizard, Vishal Toora’s breakdown of what’s happening with MYND’s trajectory.
—Joseph Morton