
Tetra Bio-Pharma (TBP.T) (“Tetra”) is a biopharmaceutical company focused on cannabinoid-derived drug discovery and development. The Company has an FDA and Health Canada cleared clinical program intended to bring novel prescription drugs and treatment to patients and their healthcare providers. Furthermore, Tetra’s evidence-based scientific approach has enabled the Company to develop an extensive pipeline of drug products for a range of medical conditions. Some of these conditions include pain, inflammation, and oncology.
In total, Tetra’s cannabinoid-derived medicines target medication indications representing addressable markets of more than $20 billion, supported by the urgent need for non-opioid alternatives. How urgent are we talking? According to the Government of Canada, 22,828 apparent opioid toxicity deaths have taken place between January 2016 and March 2021. More recently, 1,772 apparent opioid toxicity deaths occurred between January 2021 and March 2021. To put things into perspective, that’s approximately 20 deaths per day. Compared to the same period in 2020, the number of opioid toxicity deaths has increased by 65%.
To make matters worse, following the onset of COVID-19, opioid toxicity deaths have increased substantially. In fact, 6,946 apparent opioid toxicity deaths have occurred from April 2020 to March 2021, representing an 88% increase compared to the same time period a year prior. Since its onset, COVID-19 has exacerbated an already tragic crisis, creating a desperate need for non-opioid alternatives. This includes cannabinoid-derived drugs. That being said, Tetra has developed an extensive portfolio of products for a wide range of applications, including its proprietary drug candidates:
- QIXLEEF™ – Uncontrolled Pain
- CAUMZ™ – Uncontrolled Pain & Cancer
- ARDS-003 – COVID-19 & Cytokine Storm
- PPP-003 – Uveitis and Other Inflammatory Conditions of the Eye
- HCC-011 – Chemotherapy-Induced Nausea
In particular, Tetra’s ARDS (Acute Respiratory Distress Syndrome)-003 addresses an urgent medical need related to the treatment of sepsis. Annual sales of drugs used to treat sepsis were approximately $2.2 billion in 2019. This number is expected to rise to $5.9 billion by 2026. Sepsis drug treatments include disseminated intravascular coagulopathy, acute lung injury, and acute kidney injury. With this in mind, in addition to targeting COVID-19, ARDS-003 is a versatile drug intended to target other types of sepsis, including those induced by chemotherapy and traumatic injury.
How addressable of a market are we talking about here? Based on multiple early studies from Wuhan, China, among all hospitalized patients, 20%-42% had ARDS. Moreover, 61%-81% of these patients required ICU care. Aside from Tetra’s ARDS-003, the Company’s QIXLEEF™ has also made significant clinical progress.
Latest News

Speaking of QIXLEEF™, on November 29, 2021, Tetra announced positive initial clinical data from its ongoing Phase 2 clinical trials, REBORN©1 (“REBORN”) AND PLENITUDE©, of QIXLEEF™ for cancer pain. For context, QIXLEEF™ is Tetra’s botanical inhaled investigational new drug with a fixed ratio of THC and CBD that meets USA Current Good Manufacturing Practice (CGMP) regulatory requirements. Put simply, CGMP regulations ensure that a product is safe for use and that it has the ingredients and strength it claims to have.
“A safe and efficient therapeutic alternative that allows the reduction of opioids is critical now more than ever to support patients in their journey against pain. Preliminary data from both REBORN©1 and PLENITUDE© confirm the safety and pharmacodynamic profile of QIXLEEF™ reported in the phase I trials. The pharmacokinetic profile of QIXLEEF™ is well indicated to help manage short episodes of pain such as breakthrough pain and will offer patients and physicians a viable, safer, and non-opioid option for pain management,” commented Dr. Guy Chamberland, CEO and CRO of Tetra.
With this in mind, the REBORN trial is a head-to-head, open-label, crossover phase 2 study against oral opioids in the management of short and frequent episodes of breakthrough pain in patients with cancer. More specifically, the REBORN trial will evaluate the preliminary efficacy of QIXLEEF™ with regards to the onset of pain relief and pain intensity compared to three types of immediate-release oral opioids. These include moral morphine sulfate immediate release, oral hydromorphone immediate release, and oral oxycodone immediate release.
On the other hand, the PLENITUDE© trial is a randomized double-blind phase 2 study assessing the safety and efficacy of QIXLEEF™ in patients with cancer who have uncontrolled pain. Both studies are being conducted across multiple clinical sites located in the United States. To date, safety data collected from the REBORN trial confirms QIXLEEF™ tolerability and a good safety profile in cancer patients with breakthrough pain. Furthermore, no serious or adverse events have been reported, only adverse drug reactions of mild intensity have been recorded.
Similarly, preliminary data from the PLENITUDE© trial also confirms QIXLEEF™ tolerability and a good safety profile in the pool of subjects treated with either QIXLEEF™ or placebo in the randomized double-blind 4-week period and in subjects treated with QIXLEEF™ during the open-label 11-month period. Overall, preliminary analysis of the data has demonstrated a positive effect on pain relief in patients treated with QIXLEEF™. However, due to regulatory compliance, Tetra cannot disclose further data on efficacy.
Breakthrough Pain
Despite the severity of the ongoing opioid crisis, opioids are heavily relied upon for the management of cancer pain. However, current opioid approaches to managing breakthrough pain are inadequate to timely relieve pain in patients with cancer. While episodes of breakthrough cancer pain typically peak within 3-15 minutes and last up to an hour, immediate-release oral morphine produces pain relief in 30-45 minutes, with effects peaking at 1 hour or more. Not great.
In addition to having an inappropriate time window of efficacy, immediate-release oral opioids also affect a patient’s functioning and quality of life, often inducing constipation, drowsiness, and sleep disorders as well as severe side effects such as respiratory depression. Furthermore, more potent opioids, such as fast-onset opioids, have a higher risk of abuse potential, increasing the risk of misuse and overdose.
According to the National Institute of Drug Abuse, more than 47,000 Americans died as a result of an opioid overdose, including prescription opioids, heroin, and illicitly manufactured fentanyl. In the same year, an estimated 1.7 million people in the United States suffered from a substance use disorder related to prescription opioid pain relievers.
In total, roughly 21%-29% of patients prescribed opioids for chronic pain misuse them. Additionally, 8%-12% of people using opioids for chronic pain develop an opioid use disorder. With this in mind, there is currently no therapeutic solution that offers fast onset of pain relief with an acceptable safety profile and low risk of abuse potential. That being said, Tetra’s cannabis and cannabinoid-derived product pipeline has the potential to play a pivotal role in opioid use reduction, thereby addressing the current opioid crisis.
Financials
According to Tetra’s Q3 2021 Financial Results for the nine months ended August 31, 2021, the Company had cash of CAD$8,571,863 on August 31, 2021, compared to CAD$2,500,612 on November 30, 2020. On August 31, 2021, Tetra had total assets and total liabilities of CAD$39,986,970 and CAD$6,371,554, respectively. In total, for the three months ended August 31, 2021, the Company reported a net loss of CAD$5,774,901 on August 31, 2021, compared to CAD$6,548,315 on August 31, 2020.
As of August 31, 2021, Tetra had 207,684,829 outstanding warrants with an average weighted exercise price of $0.44. Upcoming, the Company has 6,900,000 warrants with an expiry date of November 30, 2021, and an exercise price of $1.29. As of August 31, 2021, Tetra had 5,577,250 stock options outstanding with a weighted average exercise price of $0.41. During the nine months ended August 31, 2021, a total of 80,000 stock options were exercised for gross proceeds of $16,800 at an exercise price of $0.21. On the contrary, on August 30, 2021, 200,000 stock options, valued at $49,457, previously granted to a consultant of the Company expired.
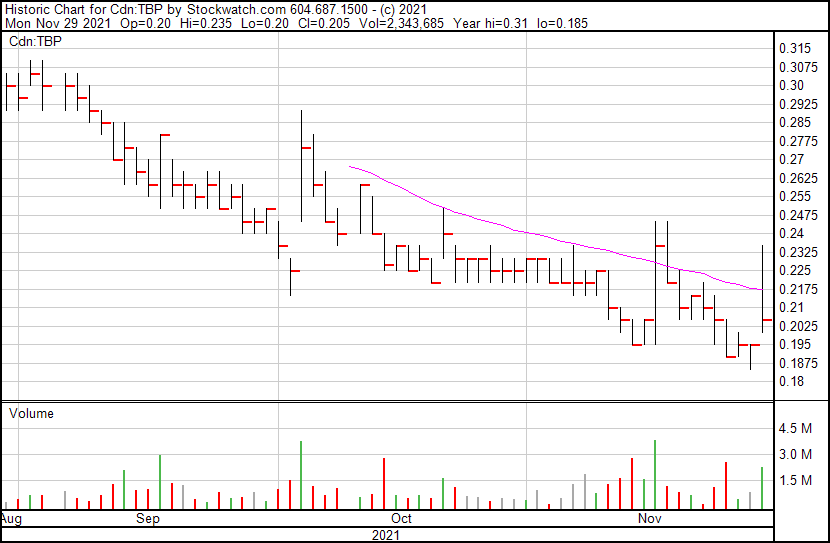
Tetra’s share price opened at $0.20, up from a previous close of $0.195. The Company’s shares are up 5.13% and were trading at $0.205 as of 11:15 AM ET.






Excellent article very well describe. We need more voices to know who tetra bio pharma is… there story is an amazing journey to bring new healing to the world.
Excellent article. Takes a long time but could be the spear point in revolutionizing pain management as the medical world knows it right now.