Cognetivity Neurosciences (CGN.C) is a technology company that has developed a cognitive testing platform for use in medical, commercial, and consumer environments. Dubbed the Integrated Cognitive Assessment (ICA) tool, Cognetivity’s cognitive testing platform is a five-minute, computerized cognitive assessment, delivered on iPad devices. Furthermore, the ICA provides a variety of benefits to clinicians and patients such as improved convenience compared to traditional pen-and-paper-based tests.
In addition to being highly sensitive to early-stage cognitive impairment, the ICA also avoids cultural and educational biases. As a result, the ICA is able to prevent a learning effect upon repeat testing. For context, a learning effect refers to a positive or negative effect from an intervention that only becomes pronounced after a certain time has passed. The effect might gradually increase with time. Moreover, the ICA is capable of supporting remote, self-https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered testing at scale and is geared towards seamless integration with existing electronic health record (EHR) systems.
That being said, on May 4, 2021, Cognetivity entered into a partnership with InterSystems, a world-leading data and interoperability platform provider. According to the terms of the agreement, InterSystems will assist the Company in the seamless integration of the ICA with additional EHRs, enabling the efficient adoption of Cognetivity’s technology in healthcare systems. InterSystems is an optimal partner for Cognetivity with its impressive network of healthcare systems in the United Kingdom (UK) and the United States (US).
This includes over 100 trusts and health boards in the UK and Ireland as well as thousands of hospitals in the US. With this in mind, Cognetivity’s ICA has already received European regulatory approval as a CE-marked medical device. In case you didn’t know, a CE-marking verifies that the manufacturer or importer affirms that its product conforms with European health, safety, and environmental protection standards. As a CE-marked device, the ICA has been deployed in both primary and specialist clinical care in the UK’s National Health Service (NHS).
Latest News

Cognetivity announced today (October 20, 2021) that it has received notification from the US Food and Drug Administration (FDA) that its 510(K) submission for its ICA has been reviewed and found to meet requirements of regulations 21 CFR 882.1470; Class II Medical Device. I’m not a robot, so most of these numbers don’t make any sense to me. If you’re in the same boat, here’s a quick rundown on the aforementioned terms. For starters, a 510(K) is a premarket submission made to the FDA to demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device.
21 CFR 882.1470 is a bit more complicated. 21 refers to Title 21 in the Code of Federal Regulations (CFR). On the other hand, 882.1470 refers to a subpart within Title 21, namely the Computerized Cognitive Assessment Aid. According to the CFR, this is defined as a prescription device that uses an individual’s score on a battery of cognitive tasks to provide an interpretation of the current level of cognitive function. ICA fits this bill quite well, so it’s no wonder Cognetivity has received approval from the FDA to market its ICA as a medical device across the US.
“We’re delighted to have reached this major company milestone, which is the culmination of many years of hard work. This grants us access to the world’s largest healthcare market, where, sadly, there is much more to be done to tackle the massive problem of dementia. Of course, we’re excited about the opportunity to revolutionize the way cognitive impairment is assessed and managed in the US and make a positive impact on the health and wellbeing of millions of Americans,” said Dr. Sina Habibi, Cognetivity’s CEO.
Having received approval, registration with the FDA will enable Cognetivity to access the vast US healthcare market, which is the largest of any country in the world. As of 2019, US national healthcare expenditure reached USD$3.8 trillion and is estimated to reach USD$6.2 trillion by 2028. Alzheimer’s and other dementias make up a substantial proportion of this figure, costing the nation USD$355 billion in 2021, according to the Alzheimer’s Association. Keep in mind, this figure could grow to more than USD$1.1 trillion by 2025, by which time the number of Americans aged 65 and older living with Alzheimer’s is expected to have more than doubled to 12.7 million.
According to Research and Markets, the Alzheimer’s diseases diagnostic and therapeutics market was valued at roughly USD$6.632 billion in 2020 and is expected to reach approximately USD$9.073 billion in 2026, expanding at a Compound Annual Growth Rate (CAGR) of 5.36% during this period. That being said, there is an enormous demand for improvements in the diagnosis and treatment of various cognitive conditions, including Alzheimer’s. Looking forward, the Company intends to execute a vigorous business development strategy to achieve a US-wide commercial rollout, including the establishment of regional outposts in the coming months. This will build on Cognetivity’s existing networks and recent successes in being selected to join two of the most prestigious and competitive accelerator programs in the US:
- TMCx Accelerator – a healthcare accelerator dedicated to identifying breakthrough healthcare technologies and enabling their subsequent commercialization (Texas Medical Center)
- Silicon Valley Health Batch 13 – an open innovation platform aiming to drive innovation globally by connecting the largest corporations with the world’s best start-ups. Its network includes more than 500 world-leading corporations, hundreds of venture capital firms, universities, and government agencies across multiple industries (Plug and Play)
Commentary
In communication with Cognetivity, Equity Guru had the privilege of receiving commentary from Dr. Tom Sawyer, the Company’s COO. When asked how receiving approval from the FDA to commercialize its ICA in the US would impact Cognetivity’s revenue potential, Dr. Sawyer stated:
“This massively positively impacts our revenue potential as it opens up the world’s largest healthcare market and allows us to market our current products in the US. This then adds to our early inroads in the UK (through the NHS) and Middle East markets, and we can now begin our commercial expansion in North America with the right regulatory approval in place.”
Lastly, when asked what other regions Cognetivity has considered for the deployment of its ICA, Dr. Sawyer commented:
“We are already active in the UK and the Middle East, but the company’s technology lends itself very well to use anywhere in the world as its usability, and importantly lack of bias caused by language or culture means it can easily be employed anywhere in the world. There is considerable potential in certain Asian markets for example, where there are tech-savvy, aging populations who are becoming increasingly motivated to look after their brain health and are happy to do this using smartphone and tablet devices, which helps with the adoption of technologies such as ours. But the bottom line is that the technology is so broadly usable and applicable that it can be deployed anywhere, and we have a clear strategy to start to address these in the near future.”
Financials
According to Cognetivity’s Interim Financial Statement for the six months ended July 31, 2021, the Company had a cash position of CAD$525,224 compared to CAD$1,359,851 on January 31, 2021. As of July 31, 2021, Cognetivity’s total assets and total liabilities were CAD$1,175,978 and CAD$1,766,391, respectively. The Company reported a net loss of CAD$1,901,412 on July 31, 2021, compared to $CAD820,220 year-over-year (YOY). Recently, on July 31, 2021, 50,000 stock options were exercised at $0.43 each for a total of $21,500. In relation to the exercise of the stock option, the fair value of $16,818 was allocated from reserves. On the same date, 208,349 warrants were exercised at $0.25 each for a total of $52,087. In relation to the exercise of the warrants, the proportionate fair value of $854 was allocated from reserves. It is worth noting that on May 8, 2020, the Company granted 332,000 RSUs to a consultant of the Company, in which 332,000 RSUs can be converted into common shares after May 1, 2022, and will expire on September 30, 2023.
“The benefits of reaching this milestone will extend far beyond the US itself…The FDA is the global exemplar in medical regulation; its name carries great weight all over the world. Without a doubt, this mark of certification will bolster our regulatory and commercial efforts in other international jurisdictions as we continue to pursue our ambitions for deployment on a truly global scale,” explained Dr. Habibi.
Cognetivity is commercializing the ICA in medical and corporate markets in the US, Europe, the Middle East, and elsewhere. As a whole, the global cognitive assessment and training market is expected to reach $11.4 billion by 2026, expanding at an impressive CAGR of 26.6%. With this in mind, FDA registration will not only assist Cognetivity’s US commercial rollout, but it will also enable the Company to more effectively expand its presence in other parts of the world as well. Ultimately, if you’re looking to dip your toes in the rapidly expanding cognitive assessment market, Cognetivity is a company that may be worth keeping an eye on as it continues to expand globally.
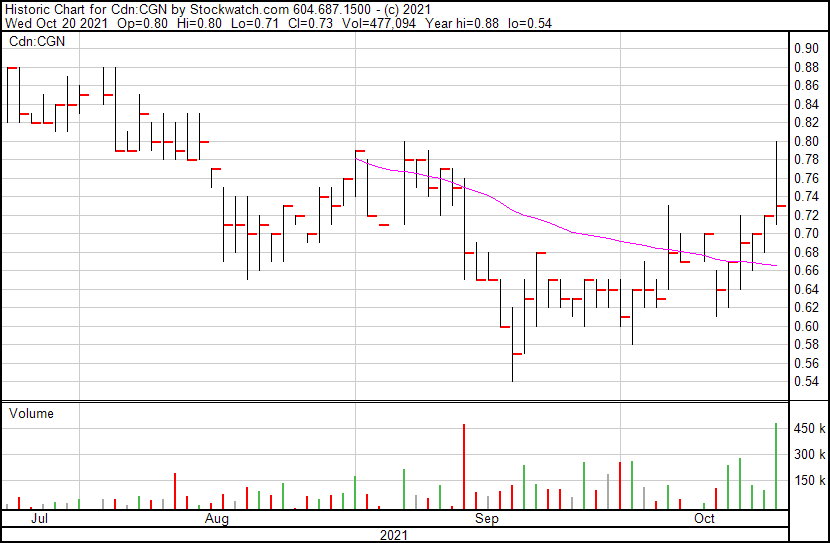
Cognetivity’s share price opened at $0.80, up from a previous close of $0.70. The Company’s shares are up 4.29% and were trading at $0.73 as of 12:05 PM ET.