On July 28, 2021 Xphyto Therapeutics (XPHY.C) announced that its acquisition target, 3a-diagnostics GmbH, has identified the first saliva-activated in-mouth biosensor candidates for the detection of a COVID-19 infection.
Xphyto Therapeutics is a bioscience accelerator focused on next-generation drug delivery, diagnostic and new active pharmaceutical ingredient investment opportunities.
3a-diagnostics is currently a commercial partner of Xphyto.
Last week, Xphyto announced that it has signed a definitive agreement for the acquisition of 3a-Diagnostics.
“We strongly believe that this acquisition will result in powerful synergies and further feed Xphyto’s diagnostic pipeline, supporting our long-term commercial growth plans,” stated Xphyto CEO Hugh Rogers.

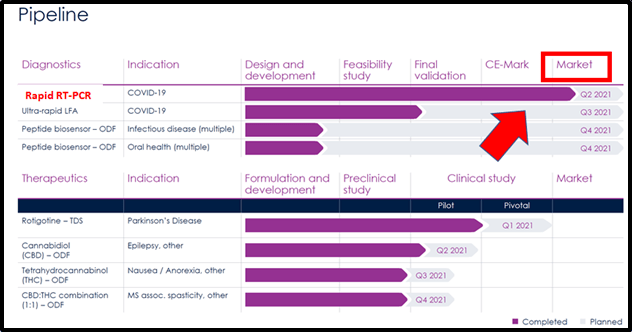
On March 18, 2021 3a-diagnostics, announced that it had received approval for its point-of-care Covid-19 rapid RT-PCR test system (Covid-ID Lab) for use in Europe.
After receiving their ISO certification, XPhyto and 3a’s Covid-ID Lab became registered within the European Union as a commercial in vitro diagnostic (CE-IVD) test.
Xphyto sent out thousands of tests to potential distributors.
The Covid-ID Lab will allow people to receive rapid test results, getting their results back after as quick as 25 minutes, and has a 95% confidence interval.
“With a sample collection to result time of 25 minutes, Covid-ID Lab combines the speed of a rapid screening test with the accuracy of a PCR diagnostic,” stated Hugh Rogers, CEO and Director of Xphyto. “Covid-ID Lab is designed for point-of-care testing, particularly in satellite and small-scale labs, such as transportation hubs, borders, care facilities, schools, pharmacies, and hospitality settings.”
Xphyto/3a’s bio-technology is desperately needed at the Vancouver airport.
This writer’s Chinese wife arrived in Vancouver 8-days ago, from Guangzhou, China. She is double-vaxxed with the Chinese vaccine recognised by the World Health Organisation (WHO) but not the Canadian Government.
At the international arrivals lounge, my spouse was tested by a Life Labs technician in the area normally reserved for seating.

After that, she was required to quarantine in a government-sanctioned hotel for 3-days.
When the negative test results came in 24-hours later, she was allowed to come home with me.
That one-night cost $1,200 (Vancouver hotels don’t give refunds for quarantine-guests). If she had Covid-19, she would’ve put her driver, co-passengers and the hotel staff at risk.
Current Covid-19 testing status-quo: expensive, tedious, risky.
The Vancouver airport is one perfect customer for XPhyto’s point-of-care Covid-19 rapid RT-PCR test system.
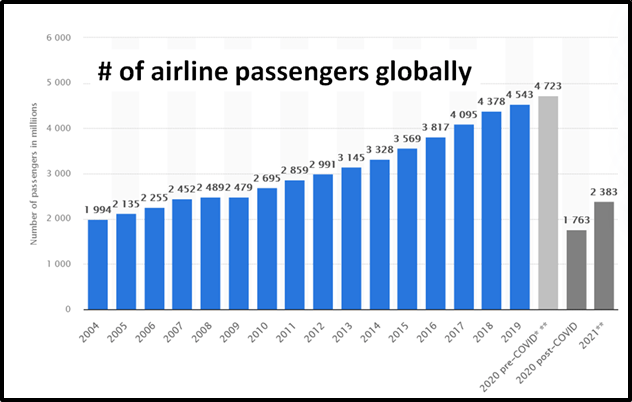
According to the Airports Council International (ACI) World Airport Traffic Report, there are currently 17,678 commercial airports in the world.
In 2021, 2.38 billion passengers are scheduled to pass through international airports.
“The enzyme-activated biosensors are developed for real-time, low-cost and easy-to-use oral screening applications for the rapid detection of infectious diseases including COVID-19 at home or at the point of care,” stated Xphyto on July 28, 2021.
A comprehensive review of current COVID-19 detection methods from lab-care to point-of-care diagnosis published July 24, 2021, in the journal Science Direct, points out the significance of biosensor technology.
Science Direct Key Findings:
- Conventional techniques for coronavirus detection like CT (computed tomography) scan, PCR (polymerase chain reaction), sequencing, CRISPR (clustered regularly interspaced short palindromic repeats), ELISA (enzyme-linked immunosorbent assay), LFA (lateral flow assay) and LAMP (loop-mediated amplification) are not sufficient to meet all testing requirements.
- There is an urgent global need for rapid, accurate and low-cost detection systems and the requirement to screen and rapidly identify current infectious disease and future pandemic threats lead scientists to recognize the need to advance new technologies.
- Biosensors in general, and 3a’s innovative biosensor system in particular, are a promising and reliable platform technology for accurate, early diagnosis and screening of infectious disease and offer advantages over traditional detection methods.
“We are delighted to announce the successful identification of the first biosensor candidates to diagnose COVID-19, which allows us to expand our portfolio of COVID-19 diagnostics in the future and to complement our recently launched rapid 25-minute PCR test, COVID-ID Lab,” stated Dr. Heinrich Jehle, managing director of 3a-diagnostics GmbH.
“After optimization, we can assess the clinical performance of our new candidates and proceed with the commercial development of this novel screening test product,” continued Jehle, “This is a major step forward in the development of next-generation COVID-19 tests, and we are optimistic that development will lead to new, low-cost, rapid, reliable and easy-to-use diagnostic options for low-threshold monitoring of the ongoing pandemic.”
“We believed in 3a’s research and development plan from the beginning, when we signed the first collaboration contract in 2020,” added Wolfgang Probst, director and chief operating officer of Xphyto, “Now that Xphyto has announced the pending acquisition of 3a, we are particularly excited about this development milestone”.
In this July 27, 2021 video, Equity Guru boss Chris Parry talks to Xphyto Therapeutics (XPHY.C) CEO Hugh Rogers about covid detection technology and the Xphyto’s psychedelic pipeline.
As we learn from the above video, Xphyto is an extremely diversified bioscience company.
Xphyto plans to acquire all of the outstanding shares of 3a for 400,000 euros, to be paid immediately, and 3.5 million euros, to be paid on closing, planned for on-or-around October 31, 2021.
- Lukas Kane
Full Disclosure: Xphyto is an Equity Guru marketing client.







