MindMed (MMED.NE, MNMD.Q) announced the start of a Phase 1 trial to assess the safety, pharmacokinetics and pharmacodynamics of DMT.
DMT, properly known as N,N-Dimethyltryptamine is a psychoactive chemical substance that occurs in many plants and animals. It is an active ingredient in ayahuasca and has often been an important part of many shamanic rituals.
MindMed’s clinical trial is being conducted as an investigator-initiated study by Dr. Matthias Liechti as part of their ongoing collaboration with the University Hospital Basel Liechti Lab (MindMed has also conducted dosing trials for both LSD and MDMA at the UHB Liechti Lab). The Phase 1 clinical trial has received all necessary regulatory approvals in Switzerland and subject enrolment has begun.
The study will explore the effects of intravenously https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered DMT, which is typically smoked or taken as a tea in the form of ayahuasca. Smoking has been the preferred method for researchers as inhalation provides faster activation than ingestion. However, taking DMT intravenously will allow them to preserve the fast activation while simultaneously allowing them greater control over the dosage. MindMed hopes the intravenous DMT https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration will allow them to provide a stable and prolonged DMT experience, while also allowing them greater control of the patient experience by enabling an acute termination of the psychoactive effects of DMT.
“We are very excited to start this study with Professor Matthias Liechti and University Hospital Basel. Currently no study has validly determined the elimination half-life of DMT or other pharmacokinetic parameters and our study will provide valuable information for future research on DMT as a tool to examine alterations of the mind. MindMed is exploring a number of psychedelic compounds as part of our mission to discover, develop and deploy psychedelic-inspired medicines and therapies to address mental illness and addiction. Our data driven approach drives our strategic choices for the development of both classical psychedelics and the very promising next generation novel chemical entities,” stated Dr. Miri Halperin Wernli, Executive President of MindMed.
This Phase 1 clinical study is aiming to assess the safety, tolerability and dose-response to DMT, and MindMed hopes it will facilitate a future Phase 2 study. The Phase 1 study will be a randomized 5-period crossover, double-blind, placebo-controlled design with 30 subjects involved.
Although psilocybin has become the most popular psychedelic amongst therapeutic companies, DMT has a pre-carved out niche, which is that the trip is significantly shorter than other psychedelics. A typical DMT trip lasts between 30 and 45 minutes, which makes it easier to research and observe patients, compared to the 6-8 hour trips usually associate with psilocybin. The shorter trip session also makes it easier for trip guides to stay with their patients. The shorter trip is certainly seen as a big benefit for DMT’s research potential.
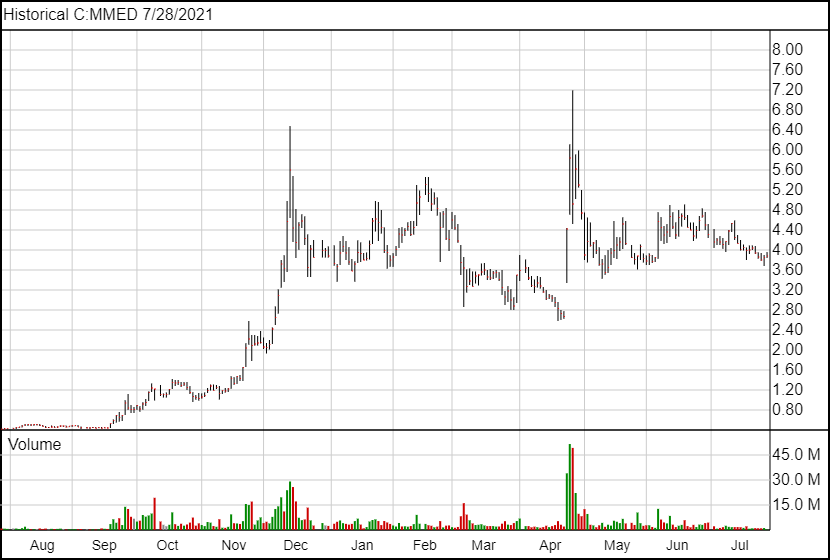
Following today’s news, MindMed shares are up 3 cents and are trading at $3.88.