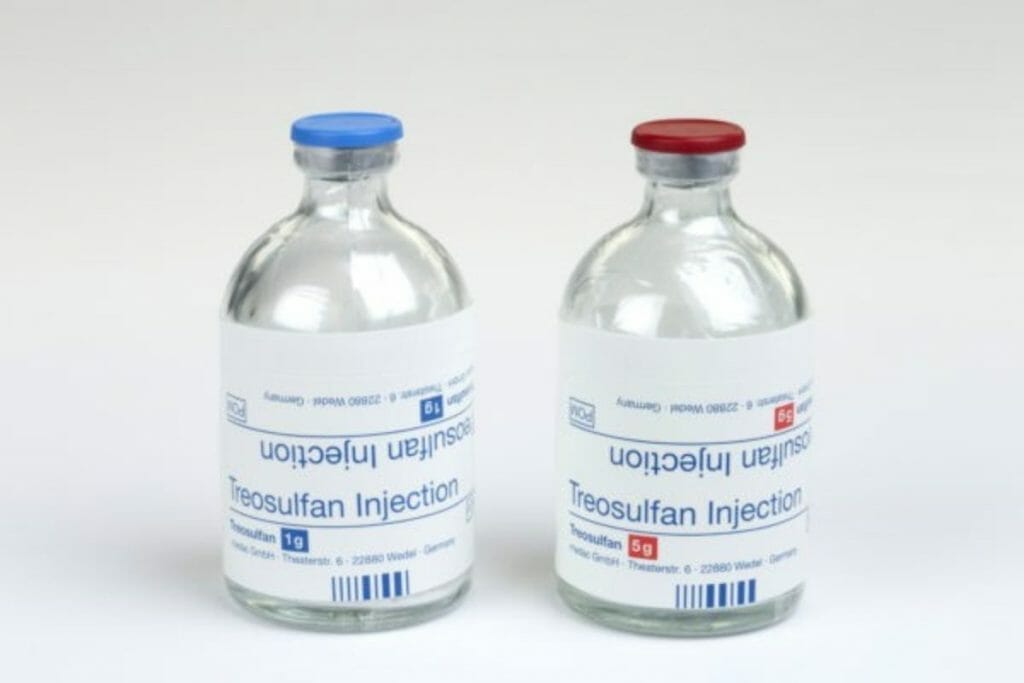
Medexus Pharmaceuticals (MDP.T) received Health Canada’s Notice of Compliance to commercialize treosulfan today, according to a press release.
Treosulfan will be sold in Canada using the brand name Trecondyv®. It’s used in combination with another drug called fludarabine as part of a conditioning treatment prior to allogeneic hemapoietic stem cell transplantation. The treatment clears the bone marrow to make space for the transplanted bone marrow cells, so they can do the work of producing healthy blood cells.
“The approval of treosulfan will provide Canadians suffering from AML and MDS with a treatment option that has shown reduced toxicity and improved survival outcomes compared to standard myeloablative regimens. With Gleolan®’s approval last September, this important milestone marks the second Health Canada approval in the last ten months, reinforcing our commitment to bringing life saving medications to the North American markets. We expect to launch Trecondyv® commercially within the third quarter this year and will continue to supply the drug to patients via the Special Access Program until then,” said Kerry Bakewell, vice president of specialty markets for Canadian operations.
Medexus Pharmaceuticals is a North American drug company specializing in finding treatments for rare diseases. Their focus is on auto-immune diseases, hematology and allergies. They have been distributing treosulfan in Canada under a Special Access Program, the authorization for which came about in March of 2019. This recent decision will make Trecondyv® available for commercial sale to treat adult patients with Acute Myeloid Leukemia (AML) or Myelodysplastic Syndromes (MDS), and who are at increased for standard conditioning therapies. Also pediatric patients older than a year with AML and MDS will be eligible.
The company is in the closing stages of extending its licensing with medac GmbH to include treosulfan for Canada. Medac is to take care of the manufacturing and supply for the drug, while Medexus will handle sales and marketing.
Medexus is up $0.27 today and closed at $7.27.
—Joseph Morton