Cybin (CYBN.NE) announced today that it has completed its 51st pre-clinical study as it continues to progress its proprietary psychedelic molecules into Investigational New Drug (IND)-enabling studies.
“These experiments greatly expand our understanding of the potential therapeutic value of the studied compounds and further demonstrate Cybin’s strong research and development capabilities,” said Doug Drysdale, Cybin’s CEO.
The Cybin Research and Development team has completed its 51st in-vitro and in-vivo evaluation of Cybin’s growing number of psychedelic compounds intended for application in therapeutics for a number of mental health objectives, including Major Depressive Disorder (MDD), Alcohol Use Disorder (AUD), and anxiety. With this in mind, more than 50 novel compounds have been evaluated via collaborations with experienced Contract Research Organizations for pharmacokinetic properties, metabolic stability, receptor binding, and safety in order to identify candidates for further development.
Currently, 1 clinical candidate, CYB001 and 3 development candidates, CYB002, CYB003 and CYB004, from the tryptamine family, have been nominated and are advancing towards clinical evaluations. Announced on May 18, 2021, CYB001 received approval from the Institutional Review Board (IRB) at Jamaica’s University of the West Indies Hospital for the commencement of its Phase II clinical trial. This study is intended to demonstrate the potential benefits of CYB001 in treating patients suffering with MDD, however, commencement of this clinical trial is subject to final confirmation of study material specifications by Jamaica’s Ministry of Health.
The trial itself will consist of a Phase IIA study of 40 patients to identify the bio-equivalent dose of CYB001 formulations versus a 25mg oral capsule. Compared to an oral capsule, CYB001’s sublingual delivery system is designed to enable rapid absorption of molecules into the bloodstream via the mouth, resulting in faster onset of action, shorter treatment duration, and lower effective dose. If the Phase IIA study is successful, Cybin plans to study the safety and efficacy of CYB001 in a randomized, placebo-controlled Phase IIB study, in 120 patients with MDD.
Moreover, Cybin recently announced on June 16, 2021, that it had selected social anxiety disorder (SAD) and generalized anxiety disorder (GAD) as the initial target indications for CYB004. SAD is estimated to affect between 3% to 7% of the US adult population. As a result, the US SAD market is estimated at USD$165 million with a global estimate of $1.15 million as of 2021. Similarly, the global GAD market is estimated at approximately $2.99 billion and is projected to grow to $4.5 billion by 2027.
With this in mind, Cybin’s formulations have positioned the Company in some of the largest mental health related markets in the world. With CYB001 Phase IIA and Phase IIB clinical trials on the horizon, investors should keep a close eye on Cybin.
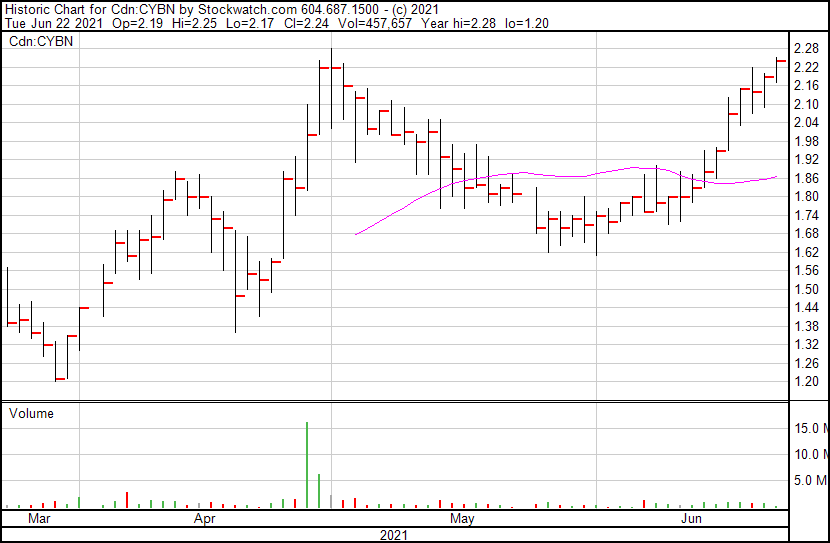
Cybin’s share price opened at $2.19, up from a previous close of $2.17. The Company’s shares are up 3.23% and are currently trading at $2.24 as of 11:02AM ET. This indicates that there has been some noticeable change following the news.
Full Disclosure: Cybin is a marketing client of Equity Guru.