On May 31, 2021 Aequus Pharmaceuticals (AQS.V) reported financial results for Q1, 2021.
Aequus is a $19 million pharmaceutical company focused on developing and commercializing high quality, differentiated products.
AQS is building a Canadian commercial platform through its own development pipeline and through license-acquisition.
On November 20, 2020 Equity Guru’s Chris Parry spoke with Aequus’ CEO and Chairman Doug Janzen about the core business philosophy of the company.
“We look at products that are approved in the US or approved in Europe,” explains Janzen, “So they’re already on market, and we bridge them into Canada.”
“Wall Street did not wake up today, wondering where that next $6 million Canadian product is,” added Janzen, “Pfizer probably spent $6 million in coffee already, at this point of the day. If we can generate 6 million revenue, the margins on that could be 80%, that’s a good business. You cobble four or five of those together, you now have a profitable business”.
“We have a good presence in ophthalmology, we have good relationships with the clinicians in that space. We’re achieving economies of scale, because we have multiple products that service their needs.”
Three years ago, “a well-known glaucoma specialist in Toronto told me, ‘If you could give me a preservative-free, single, multi-dose bottle, I would use that version’. We listened. This is how we do our business. We listen to clinicians.”
“We went out and we found a company in the UK, that makes a big line of preservative free eye care products, and delivers them in easy-to-use multi dose bottles”, added Jansen.
On March 1, 2021 AQS announced the commercial availability of Evolve preservative free lubricating eye drops for dry eye care.
The products will be available exclusively for sale by eye care clinics in Canada, where patients can receive the full benefit of dry eye treatment plans when they are diagnosed, prescribed, and monitored by eye care professionals.
Dry Eye Disease (DED) is a common disorder of the tear film that leads to ocular surface damage over time, it affects approximately 6.3 million Canadians.
Home confinement, e-learning and working from home due to the COVID-19 pandemic means spending more time looking at screens, which has a significant impact on eye health.
A combination of reduced frequency and intensity of blinking during screen time increases the risk of inducing or exacerbating dry eye disease.
In general, two objects that repetitively move against each (chain/sprocket, piston/cylinder, eyeball/socket) need to be lubricated to avoid degradation of the contacting surfaces.
Bicycles use chain-oil, internal combustion engines use 10W30.
A healthy eye lubricates itself by producing tears at a slow and steady rate, keeping itself moist and pain-free.
A person suffering from Dry Eye Disease (DED) may be experiencing hormonal changes, side effects from medication or some other factor, causing the eye to either – not produce enough tears, or omit parts of the tear that promote lubrication.
If you are one of those people, you know that DED can be a form of torture.
“Guys this quite depressing and pls help me out It’s been 2 days I have started seeing rainbow rings and starburst on lights,” states one sufferer on DED forum.
“Could someone please put me out of this misery and tell me how to find affordable relief?” asks another DED sufferer, “I can barely keep my eyes open and I’m in the middle of nursing school. I’m desperate!”
The Evolve line of dry eye products “will not only improve treatment and compliance for better patient outcomes but create an improved revenue stream for professionals who spend additional time and resources treating dry eye patients in practice,” states Grant Larsen, Chief Commercial Officer for Aequus Pharmaceuticals. “This launch adds another 2 products to our growing Eye Care portfolio and represents a significant revenue opportunity for Aequus going forward.”
The Evolve lubricating eye drops are crafted specifically for supporting optimal eye care and contain:
- Preservative and phosphate free formulations
- A secure antimicrobial twist-cap
- Compatibility with soft and hard contact lenses
- 350+ micro-sized drops per bottle – the highest number of drops on the market
- Non-blurring formulations
- Patented PureFlowTM Technology, and blue aiming tip
- Soft sides for easy drop dispensing

“We had a productive start to 2021,” confirmed Janzen in the May 31, 2021 financial release, “Despite the many COVID-19 related restrictions that impacted all of Canada, our sales force continued to find creative ways to manage the business”.
AQS launched a new E-commerce platform www.aequuseyecare.ca, beefing up its sales infrastructure making it easier to launch new products and scale commercial operations.
“Sales were down slightly in Q1 compared to the same period last year and lower than our record quarter in fourth quarter 2020,” explained Janzen, “which from a timing perspective, appeared to cannibalize some January sales.”
“We were pleased to see sales meet internal forecasts since February and we expect to continue our revenue growth trajectory going forward,” added Janzen.
Commercial Update
- On March 01, 2021 AQS announced the commercial availability of Evolve™ preservative free lubricating eye drops for dry eye care. Availability of the product is only for Eye Care Professionals, paving the way for additional products and devices in this specialty healthcare channel.
- With the launch of Evolve, Aequus has added an E-commerce platform to order and direct ship to professionals Nationally. The company expects to expand direct to ship to patients, on behalf of professionals, or with authorization of professionals in the coming months. This will streamline supply and value to both consumer and trade channels.
- COVID has provided Aequus the opportunity to develop our CRM capabilities and database details for future marketing and deployment efficiencies. We can now segment, target, measure and analysis resource allocations in almost real time and apply these learnings to business. This agility will allow us to commercialize in multiple therapeutic areas, with targeted B2B precision.
- All sales and marketing personnel have been retrained in online/digital analysis, remote hybrid selling, video presenting and social selling best practices.
- Regarding Zimed, initial inspections have begun by Health Canada for the review of SOP’s, logistics and warehousing all received with positive comments. The full submission for approval of Zimed is on track for late June; Launch is on track.
- Currently in negotiation with Sandoz Canada Inc., AQS partner with both Tacrolimus and Vistitan branded products. Expect to extend terms on Vistitan through the end of 2021, with intent to extend longer term contracts on both brands before year end. Both brands continue to grow, gain share and deliver profitably for both companies.
- A recent study published in the British Journal of Ophthalmology compared glaucoma medications for efficacy related to lowing intraocular pressure (IOP). The results support Vistitan’s competitive advantage and value to patients, provincial health organizations and insurance payers Nationally.
Financial Update
AQS reported an operating loss before other income of $623,636 for Q1, 2021, a 53% change from the loss before other income of $408,706 in the three months ended March 31, 2020.
The higher loss in First Quarter 2021 was primarily due to lower revenue and an increase in product development and regulatory costs related to Zimed-PF as it progresses through the Health Canada review process as well as higher non-cash expenses triggered by the conversion of convertible debt to equity.
Q1, 2021 had revenues of $491,821, which is a 15% decrease over the $579,450 revenue during the First Quarter 2020. The fluctuations in revenue were primarily driven by timing differences resulting from consumer buying patterns and not believed to be attributable to any specific cause or variable controllable by the Company. COVID restrictions continue to be an access barrier in Ontario and Quebec, but the impact of the COVID restrictions is not measurable.
Sales and marketing costs in First Quarter 2021 were $477,830 when compared to $451,146 in First Quarter 2020, an increase of 6% or $26,684. The majority of the increase related to the launch of the Evolve products and related salesforce and marketing expenses.
Research and development costs in First Quarter 2021 were $87,898 when compared to $14,317 in First Quarter 2020, an increase of 514% or $73,518. The increase related to market access consultants and Health Canada approvals required for the launch of the Evolve products.
General and https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration costs in First Quarter 2021 were $547,116 when compared to $522,693 in First Quarter 2020, an increase of 5% or $24,423.
General Corporate Update
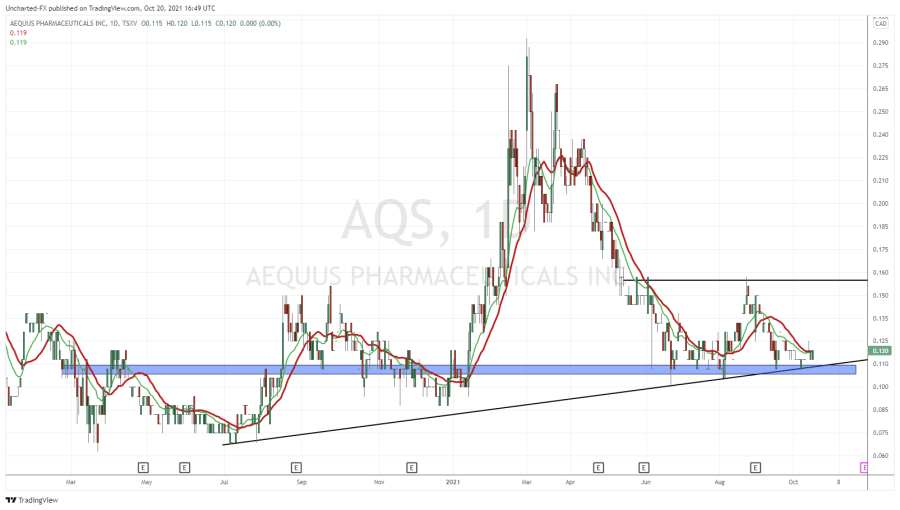
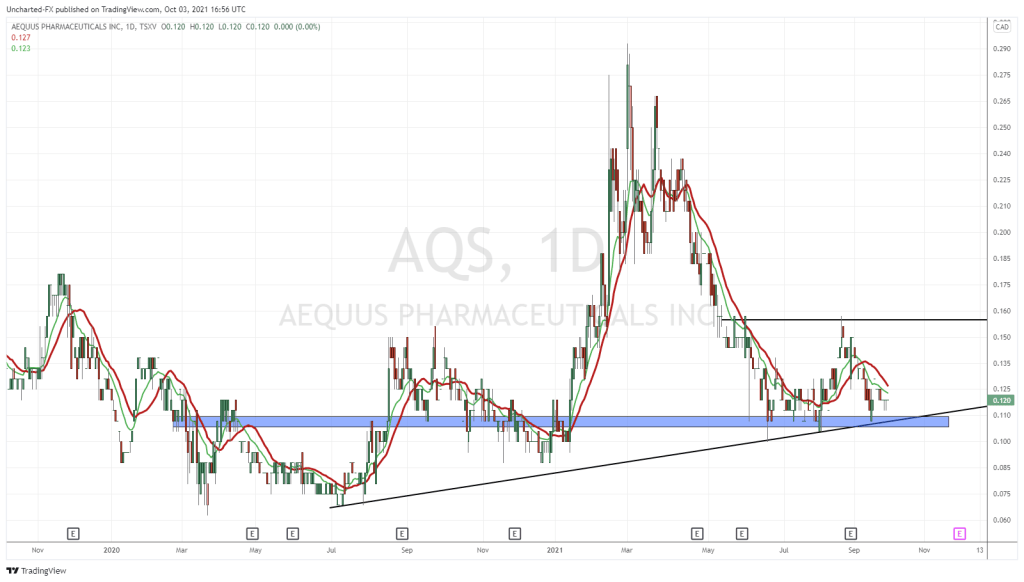
- In February 2021, AQS closed a private placement of 6,666,666 units at a price of $0.15 per unit, for proceeds of $1,000,000 to Marc Lustig, a director of the Company. Each unit shall consist of one common share and one-half warrant. Each warrant shall entitle the holder to purchase one common share at an exercise price of $0.25 for 24 months.
- In March 2021, AQS exercised its right to trigger an accelerated expiry under the terms of a warrant indenture. Between March 2 and April 1, 2021, the Company issued 12,343,750 shares at $0.12 per share, pursuant to the exercise of warrants for net proceeds of $1,481,250. The Company also issued 317,000 shares at $0.22 per share pursuant to the exercise of warrants for net proceeds of $69,740.
Planned Shelf Prospectus Filing to Replace Soon to Expire Existing Shelf Prospectus
“A shelf prospectus is a short form prospectus that permits companies to offer, from time to time over a 25-month period, up to a maximum aggregate dollar value of one or more types of securities (including preferred shares, common shares and/or debt securities),” states Pilot Law, “The base shelf prospectus contains, or incorporates by reference, disclosure not specific to a particular offering of securities.”
AQS maintains an active Shelf Prospectus, providing flexibility to efficiently raise money when market conditions are favourable. The current Shelf Prospectus expires on October 16, 2021. AQS planning to update the Shelf Prospectus over the next few months and expects to file the preliminary short form base shelf prospectus prior to expiry of the current Shelf Prospectus.
“The AQS business model is to sidestep the long clinical trial process of getting new medical products and treatments approved by regulators, by buying and licensing existing approved but perhaps unevolved or underutilized products,” stated Equity Guru’s Chris Parry on October 19, 2020, “and reworking them to be more effective, user-friendly, and appealing to consumers”.
Currently in Canada, the dry eye market is estimated at over $9O million, which includes both prescription and over-the-counter products.
Full Disclosure: AQS is an Equity Guru marketing client