Cybin (CYBN.E) filed a new provisional patent application for its ongoing drug candidate programs today, according to a press release.
The application includes compositions which the company anticipates will improve pharmacokinetic profiles while keeping efficacy measures of the original molecules. The company’s patent also offers new delivery systems to increase onset of Cybin’s psychedelic tryptamine, while reducing side effects, and the duration of said side effects when they do happen.
“By continuing to innovate in directions to enhance the patient experience and reducing clinical observation times, we believe our treatments can decrease costs and increase the capacity of medical professionals in this field, which will promote increased access to these important therapeutics for those in need,” said Doug Drysdale, chief executive officer.
Cybin is a biotechnology company working to provide innovations for the burgeoning psychedelics space through development, research and deployment of novel drug delivery systems, novel formulation approaches and treatment regimens.
Patent Filing Highlights:
- The Company has a provisional patent application including disclosures directed to novel oral dosage forms believed to provide therapeutic advantages in addressing the Company’s target indications over the current art.
- The patent application is synergistic with other patent applications filed by the Company related to a delivery technology covering various chemically synthesized psychedelic molecules which is expected to provide faster onset times in a similar route to intravenous treatments.
- The patent application bolsters the Company’s portfolio related to deuterated psychedelic molecules and analogues which are expected to provide greater stability, better potency, more control over duration and greater bioavailability than other forms of these molecules.
- The patent application disclosures related to faster therapeutic onset, decreased duration, and decreased side effects are anticipated to greatly reduce the clinical costs of https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration resulting from reduced clinical observation time.
The patent also includes methods intended to use the compositions to promote not only faster onset and decrease side effects, but also decreased duration of action.
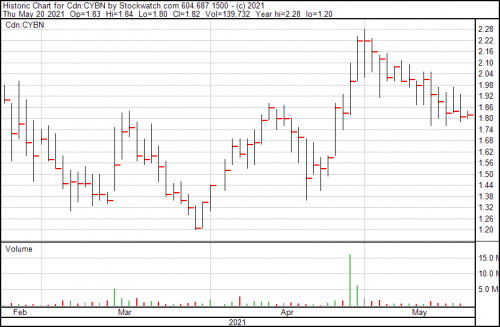
Cybin’s up a penny today, trading at a $1.82.
—Joseph Morton
Full disclosure: Cybin is an equity.guru marketing client.