On January 28, 2021 XPhyto Therapeutics (XPHY.C) announced its drug formulation and delivery business strategy and milestones for 2021.
Xphyto describes itself as “a bioscience accelerator” “targeting growth through commercialization of its product pipeline”.
That doesn’t do justice to the complexity and diversity of its central design.
Think of XPHY as a publicly-traded Swiss Army Knife.
It cuts, saws, slices, tweezes and scissors.
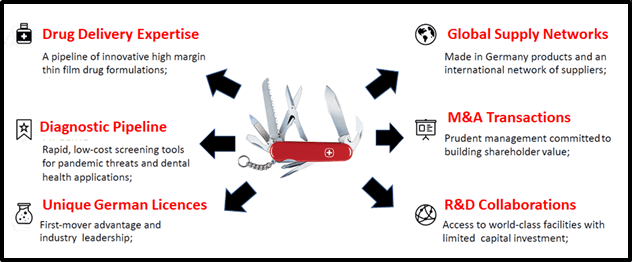
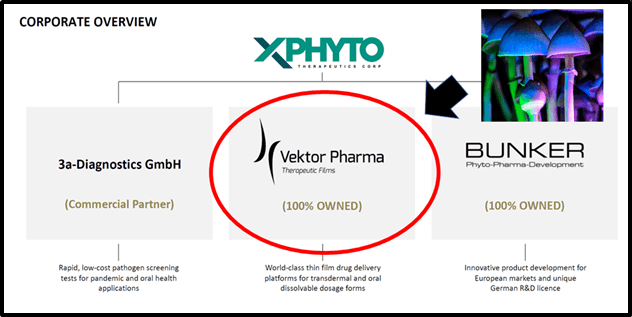
XPHY owns 100% of a German narcotic manufacturer called Vektor Pharma, another subsidiary called Bunker that has all the licenses to study cannabis as a medicine, and it has formed multiple partnerships with universities and research centers.
Vektor is located in the district of Biberach, Baden-Württemberg, Germany.
2021 Core Milestones:
- Commercialization of infectious disease diagnostics
- Clinical validation of transdermal and sublingual drug formulations
- Continued investment in psychedelic medicine
This fall, Vektor Pharma advanced two sublingual Oral Dissolvable Film (ODF) development programs to deliver cannabidiol (CBD) and tetrahydrocannabinol (THC).
Oral Dissolvable Film (ODF) sub-lingual (oral) strips are known to increase bioavailability. It’s an upstream medical technology that can be applied to a wide array of different drugs.
Three months ago, XPHY announced that Vektor had signed a research agreement with a leading German university for “the exclusive development of a proprietary biotechnology process for the industrial manufacture of psilocybin as a certified active pharmaceutical ingredient (API)”.
Psilocybin is a natural psychedelic compound produced by 200 species of fungus. The fungal mushrooms have hallucinogenic effects when eaten.
“Psychedelic agents are a promising new class of therapeutic drug,” stated Hugh Rogers, CEO and Director of Xphyto, “We see a natural opportunity to apply XPhyto’s drug formulation expertise and Vektor’s proven drug delivery platforms to these emerging APIs.”
“50 years I’ve been bumbling around this planet, and the best damn weekend I’ve had on it to date occurred recently, under the influence of mushrooms,” wrote Equity Guru’s Chris Parry on December 9, 2020.
“The carpet didn’t talk to me and the walls weren’t bleeding, and the sunrise wasn’t the incarnation of Shiva. That ain’t it, kids,” added Parry, “No, what happened is my head just opened right up and someone I cared about did likewise and a relationship went to a place it hadn’t before and everything changed going forward.”
In 2021 Vektor will “position itself for commercial manufacturing, pipeline development and drug formulations for critical mental health conditions”.
“2021 is expected to be a transformative year for Vektor. We will be building on significant momentum from 2020 as we advance our drug formulation, contract development, and psychedelic business,” stated Prof. Dr. Thomas Beckert, managing director of Vektor, “The addition of scalable in-house manufacturing capability creates the potential for significant growth across virtually all of our development and commercialization programs.”
For over a decade, the Vektor and its team have been leaders in the design, testing and manufacture of thin film drug formulations, particularly transdermal patches and sub-lingual (oral) strips for the delivery of Active Pharmaceutical Ingredients (API) for the treatment of pain and neurological conditions.
Xphyto has identified a number of psychedelic compounds that are emerging as strong potential candidates for the treatment of mental health related medical indications such as depression, anxiety, addiction, anorexia and post-traumatic stress disorder.
Commercial Manufacturing Facility
“In anticipation of Vektor’s drug product commercialization schedule, and due to significant interest in contract manufacturing, Vektor is planning to build a new commercial drug manufacturing facility in Germany in 2021,” stated Xphyto.
“Vektor has secured a property in the district of Biberach, near its current laboratory, and is currently in the due diligence process to ensure suitability of the land for construction and use as a commercial drug manufacturing and laboratory site.
The estimated maximum capacity of laboratory and manufacturing space that could be constructed on the property is 3,000 m2 (32,000 ft2). Xphyto is reviewing scalable construction options to synchronize manufacturing capacity and demand with the Company’s projected growth and timelines”.
Clinical Studies
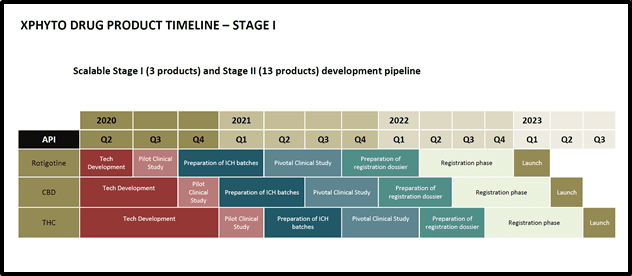
“XPhyto’s drug formulation business is focused on neurological indications with strong market demand and the potential for improved patient outcomes. In 2020, XPhyto reported significant advancements in four drug formulation development programs.
In 2021, Xphyto is planning to complete one clinical pilot study in each quarter as follows:
Q1) Rotigotine – transdermal patch in Parkinson’s disease;
Q2) CBD – oral/sublingual strip in treatment resistant Epilepsy;
Q3) THC – oral/sublingual strip in anorexia/nausea; and
Q4) CBD:THC (1:1) – oral/sublingual strip in Multiple Sclerosis associated spasticity.
Each study is planned to be carried out over an approximately two-week period as an open label, randomized, crossover, two-period, two-sequence, single dose pilot study to assess the relative bioavailability of each product.
Pending positive results, the Company plans to advance the products toward a final pivotal study and application for regulatory approval.
Development of Oral Biosensors and Contract Development & Manufacturing
XPhyto’s diagnostic portfolio includes several novel peptide-based oral screening tests, under licence from 3a-diagnostics GmbH, for the detection of infectious diseases including influenza A, and oral health indications, such as peri-implantitis.
In 2020, XPhyto confirmed successful activation of its peptide-based biosensor program when delivered using Vektor’s sublingual drug delivery platform.
In 2021, XPhyto plans to continue development and validation of its biosensor products and plans to bring at least one biosensor product to market in Europe by yearend. In-house commercial manufacturing capacity, as set out above, is expected to maximize profitability of the biosensor commercialization.
Psychedelic Transdermal and Sublingual Drug Formulations
Psychedelic compounds are a promising new class of API with potential for the treatment of mental health conditions such as depression, anxiety, addiction, and trauma-related stress disorder.
Psychedelics could provide a major improvement over currently available therapeutics for a global market with unmet medical need.
Vektor will begin planning and development work for the incorporation of various psychedelic compounds into its drug formulations.
This is facilitated by Vektor’s drug delivery platforms in combination with, XPhyto’s agreements for the development of industrial scale biotechnology processes for the production of psilocybin in Germany, and for the research and development of a broad range of psychedelic compounds, including psilocybin, mescaline, LSD, MDMA, DMT, in Canada.
XPhyto believes that the development of standardized drug formulations with precise, predictable and efficient API delivery for clinical study and therapeutic use, is critical to advancing the field of psychedelic medicine.
Equity Guru’s Jody Vance recently spoke with Xphyto’s CEO and Director Hugh Rogers to get a bird’s eye view of the company, its portfolio and what the markets can look forward to over the next 12 months.
The US Food and Drug Administration (FDA) has twice designated psilocybin as a “breakthrough therapy” for the treatment of severe and treatment-resistant depression.
It’s not difficult to see how Xphyto’s existing technology could be deployed in an arena where bio-availability and dosage-control will be paramount.
XPhyto is now securing industrial scale production of psychedelic APIs and the standardization of drug formulations for the delivery of such APIs.
We’ve known Xphyto CEO Hugh Rogers for over a year. In a sector full of flakes, he’s always stood out as the adult in the room.
These ambitious 2021 drug formulation & delivery milestones are further evidence of his substance.
- Lukas Kane
Full Disclosure: XPhyto is an Equity Guru marketing client.







