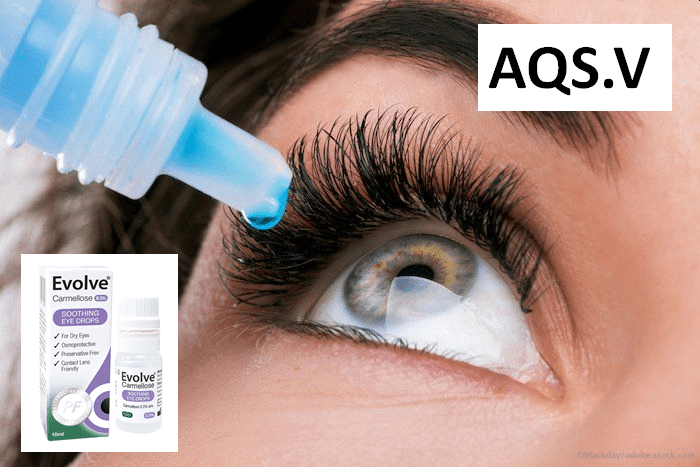
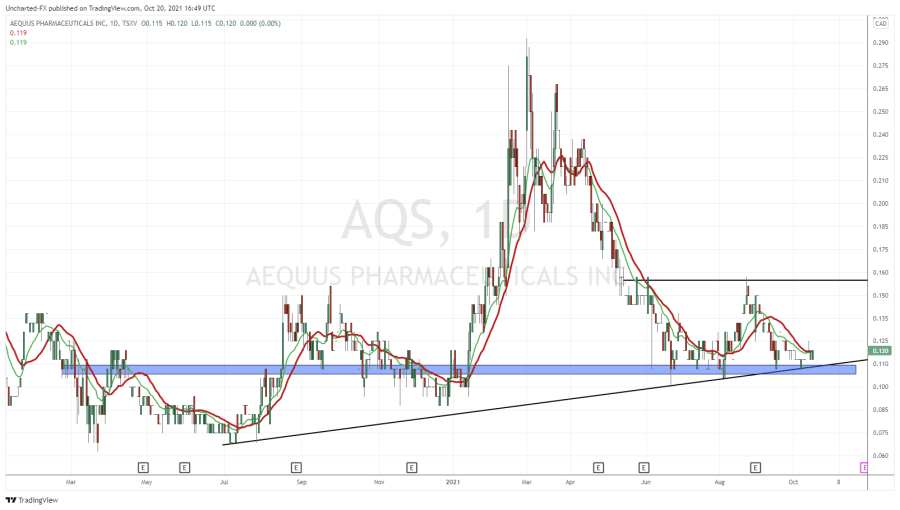
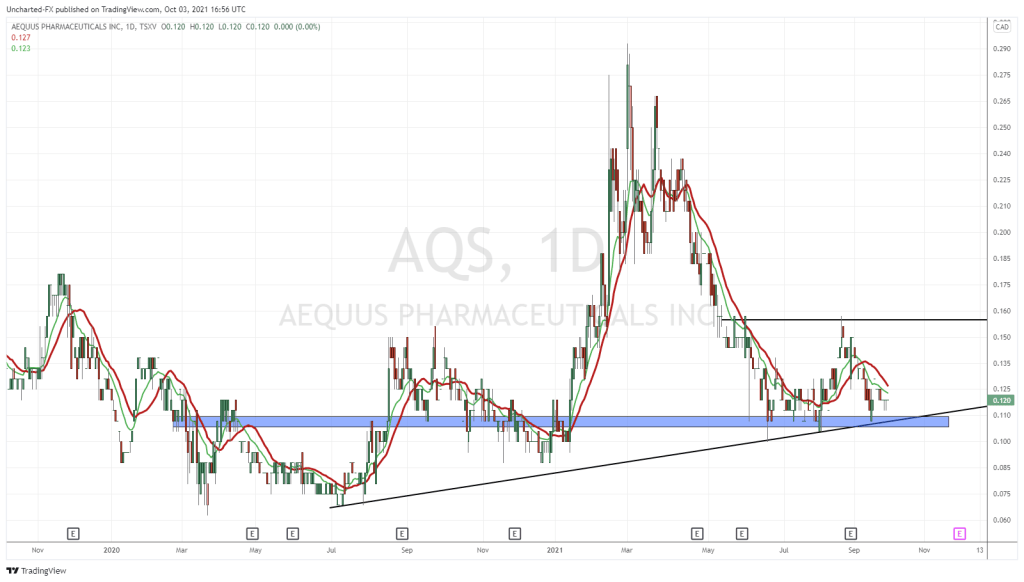
On October 29, 2020 Aequus Pharmaceuticals (AQS.V) – together with its partner Medicom Healthcare – announced that it has been “issued a new medical device licence for the second of three product submissions made for the Evolve preservative free dry eye product line”.
Aequus is a vibrant pharmaceutical company focused on developing and commercializing high quality, differentiated products.
AQS is building a Canadian commercial platform through internally-created products and through license-acquisition.
“This is an exciting approval for Aequus, as the Evolve range expands, providing much needed benefits to patients suffering from Dry Eye Disease,” stated Aequus CEO, Doug Janzen.
“Dry eye disease (DED) is a common disorder of the tear film that leads to ocular surface damage over time.
DED affects approximately 6.3 million Canadians, representing approximately 21% of the population.
Home confinement, e-learning and working from home due to the COVID-19 pandemic means spending more time looking at screens, which has a significant impact on eye health.
A combination of reduced frequency and intensity of blinking during screen time increases the risk of inducing or exacerbating dry eye disease”.
“Eye Care professionals can now offer Evolve to deliver exceptional patient experience while protecting the long term health of every patient’s eyes,” stated Grant Larsen, Chief Commercial Officer at Aequus.

In general, two objects that repetitively move against each (chain/sprocket, piston/cylinder, eyeball/socket) need to be lubricated to avoid degradation of the contacting surfaces.
Bicycles use chain-oil, internal combustion engines use 10W30.
A healthy eye lubricates itself by producing tears at a slow and steady rate, keeping itself moist and pain-free.
These tears consist of three layers: oily, watery, and mucus. Each layer has a designated role in lubricating the eyes.
The outer oily layer slows evaporation of the tear.
The middle watery layer cleans the eye and helps to wash away dust and other airborne irritants.
The inner mucus layer helps the watery layer to adhere to the eye-ball.
A person suffering from Dry Eye Disease (DED) may be experiencing hormonal changes, side effects from medication or some other factor, causing the eye to either – not produce enough tears, or omit parts of the tear that promote lubrication.
If you are one of those people, you know that DED can be a form of torture.
“Guys this quite depressing and pls help me out It’s been 2 days I have started seeing rainbow rings and starburst on lights,” states one sufferer on DED forum.
“My supervisor thinks I don’t care about my job because of how tired I look despite being in the office 12 + hours a day and weekends. My eyes will feel tired and twitch,” states another.
“Could someone please put me out of this misery and tell me how to find affordable relief?” asks another DED sufferer, “I can barely keep my eyes open and I’m in the middle of nursing school. I’m desperate!”

“The new Medical Device License was issued for Evolve Daily Intensive — an advanced formulation of 0.2% Hyaluronate, free of preservatives and phosphates, and made available in a multi-dose bottle for ease of use for all patients.
The formulation contains 350 drops that can be dispensed with gentle squeezing — an important feature for chronic users and many dry eye patients.”
Evolve Daily Intensive is the second lubricating eye drop being offered by Aequus as part of a full line of products made exclusive to Eye Care Professionals.
“These Hyaluronate drops in particular are seen as the workhorse of the entire line, and are currently the fastest growing Hyaluronate drops in the UK,” states Janzen, “The full range is expected to generate $10M a year in Canadian sales at maturity.”
Aequus is advancing launch activities the newly approved Evolve products, while a third drop in the Evolve range is currently under review with Health Canada.
Launched in 2015 in Europe, the Evolve brand has grown to 5 products across 35 countries.
With an array of products, the brand can address the various symptoms involved with dry eye disease and blepharitis including discomfort, stinging, burning, and dryness.
“The AQS business model is to sidestep the long clinical trial process of getting new medical products and treatments approved by regulators, by buying and licensing existing approved but perhaps unevolved or underutilized products,” stated Equity Guru’s Chris Parry on October 19, 2020, “and reworking them to be more effective, user-friendly, and appealing to consumers”.
Currently in Canada, the dry eye market is estimated at over $9O million, which includes both prescription and over-the-counter products.
- Lukas Kane
Equity Guru’s own Chris Parry spoke with Aequus Pharmaceuticals CEO and chairman, Doug Janzen, recently. Here are both parts of that interview. Tune in!
Full Disclosure: Aequus Pharmaceuticals is an Equity Guru marketing client.