The scientific process isn’t exactly what you were taught in grade 4. There’s a lot more to it, owing to some historical—well, let’s call them what they are—atrocities, which have taken place as long as white lab-coats have been all the rage in knowledge-defining circles. This process is expensive in both time and money and that’s inherently necessary to impose a modicum of baked-in due-diligence to the process.
Getting the right drugs to the right people at the right time isn’t a process we should rush, and while there’s definitely pressures coming from multiple different angles to find a solution to COVID-19, it’s important that we continue with the process in a measured fashion. Part of this is ethics. We need to ensure that the chemicals we’re using have been tested ethically, and if you were inclined to reward good science and better behaviour, now would be the time.
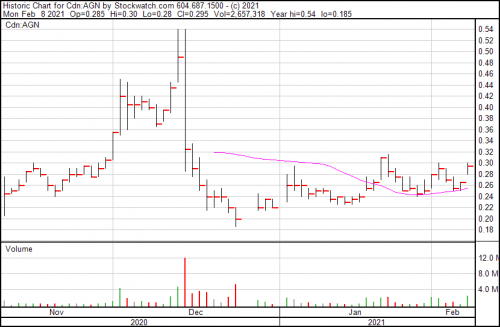
Algernon Pharmaceuticals (AGN.C) got the ethics nod from the central institutional review board for U.S. study sites for its multinational phase 2b/3 human study of NP-120 (Ifenprodil) for COVID-19 today.
This is how to invest in defeating the pandemic. Find a company doing Gods work, and throw your money at it.
If you’re scientifically inclined, Ifenprodil is an NMDA receptor antagonist specifically targeting the NMDA-type subunit 2B. If you’re not, it’s a chemical that works to inhibit a certain receptor in the brain. They’re commonly used as an anesthetic for animals and humans, and the anesthesia they induce is the dissociative kind: think more ketamine than dilaudid.
NMDA receptor antagonists are thought to have some potential for treatment in diseases like Alzheimers, Parkinsons, stroke and traumatic brain injuries. It’s potential use in COVID-19 comes in the wake of a 40-patient, 4-week trial in South Korea designed to test and determine the efficacy of the chemical in infected patients, showing severe pneumonia. The tests involved patient randomization on a 1:1 fashion, where they received either standard of care (SOC) or SOC with Ifenprodil.
The company decided to explore Ifenprodil as a treatment option for COVID-19 after discovering a research study indicating the drug was active in an animal model for H5N1, a particularly lethal strain of avian flu with a 60% death rate in humans. The study showed that Ifenprodil reduced the mortality rate by 40%, and also reduced both inflammation and injury to the lungs.
The NMDA receptor is found on many tissues, including lung cells and T-cells. Algernon’s take is that Ifenprodil can reduce the infiltration of neutrophils, a type of white blood cell that helps heal damaged tissues, and T-cells from entering the lungs where they release a neurotransmitter called glutamate, and proteins called cytokines.
COVID-19 produces something called a cytokine storm, which attacks the lungs and causes death among patients. If these neutrophils and T-cells can’t enter the lungs and release their payload of glutamate and cytokines, then there’s no cytokine storm.
The United States Food and Drug Administration has provided feedback, and the company is going to increase the size of their 2b part of the study from 100 to 150 patients. The extra fifty patients will receive an increased dose of Ifenprodil. If the data comes back positive, the company will move onto a phase 3 study. The phase 2b data will assist in determining the numbers of patients required to reach statistical significance in phase 3.
“Patients will be randomized in a 1:1:1 manner and will either be treated using an existing standard of care, or standard of care plus Ifenprodil 60 milligrams (taken as one 20 mg tablet three times daily), or standard of care plus Ifenprodil 120 mg (taken as two 20 mg tablets three times daily).
The primary end point will be the change in a patient’s clinical score using the World Health Organization’s ordinal scale. In addition, over the testing period, doctors will observe whether there is an improvement in a number of secondary end points, including mortality, blood oxygen levels, time spent in intensive care and time to mechanical ventilation.”
The company has gotten the regulatory nod from both the FDA and Health Canada, and to make sure they can get enough people enrolled in the study, the company has tapped Novotech their CRO partner, to find them two more countries to add. So Novotech has been conducting feasibility studies in both the Philippines and Romania, which are COVID-19 hotspots. There isn’t a preset number of patients assigned to each site, and the goal is to get 150 patients involved across all sites and countries as soon as possible.
The company is currently carrying a cash position of $7.5 million, which the company contends is enough money to cover the associated costs of their phase 2 IPF and chronic cough study, the investigator-led COVID-19 study in South Korea, and the multinational 2b/3 COVID study.
“We continue to get closer to dosing our first patient in our phase 2b/3 human trial investigating Ifenprodil for COVID. There is a tremendous amount of work involved and many moving parts to getting a multinational clinical trial under way once regulatory approvals are received and we will continue to update the market as we progress,” said Christopher J. Moreau, chief executive officer of Algernon Pharmaceuticals.
On the manufacturing side, the company has called upon U.S.-based research organization, Cascade Chemistry, to assist their scaling efforts for Ifenprodil. Algernon requires the active pharmaceutical ingredient (API) of Ifenprodil in order to develop it in different fashions, including injectables, and a slow release tablet. Cascade is nearly finished their first engineering run of API.
If the study turns out to be a success, the company hopes to have a finished product ready to go on shelves as soon as possible.
—Joseph Morton