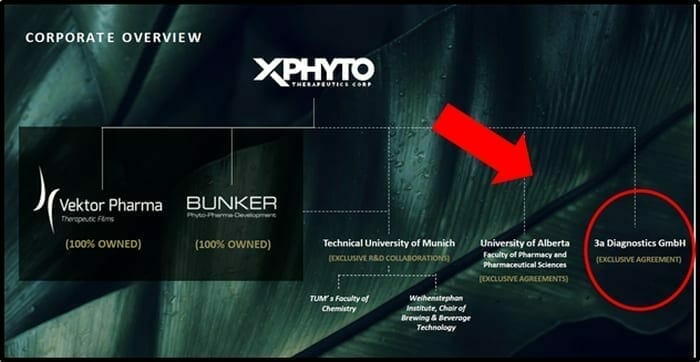
On July 6, 2020 XPhyto Therapeutics (XPHY.C) announced that its exclusive diagnostic partner, 3a-Diagnostics took a major step towards the development of an affordable, portable screening tool that will disrupt the fight against pandemic threats.
3a is a German research-based biotech that specializes in point-of-care test systems.
The July 6, 2020 news confirmed successful function of 3a’s proprietary coronavirus RNA probes, though prototype Lateral Flow Assay (LFA) testing.
For a neophyte biotech investor, a “Lateral Flow Assay” sounds intimidating, but you don’t need a PHD in chemistry to wrap your head around it.
LFAs are simple paper-based devices that detect the presence (or absence) of a target analyte in liquid sample (matrix) without the need for specialized and costly equipment.
“LFAs are designed for use at point of care/need: the physician’s examination room, emergency ward in general hospitals,” explains the Analytical and Bioanalytical Chemistry Journal.
“Especially in third-world countries these tests are used for biomedical purposes, since there is no need to refrigerate the strips,” continues the Journal, “Owing to their extended shelf life, large batches can be prepared, diminishing the variation between batches”.
LFAs are designed for disposable single use so that no contamination with a previously tested sample will occur.
The most famous application is the home pregnancy test.
In this dry, methodical video, a Huddersfield University lecturer explains LFA technology:
“Visual confirmation of test results (probe activation) was observed in five to seven minutes,” states Xphyto.
3a is focused on detection of viruses during the early stages of infection when patients are highly contagious and often asymptomatic.
Coronaviruses encode their genome using RNA (not DNA). Two viral RNA probes have been developed: 1) a COVID-19 specific RNA sequence, and 2) a universal coronavirus RNA sequence (shared by all known members of the coronavirus family).
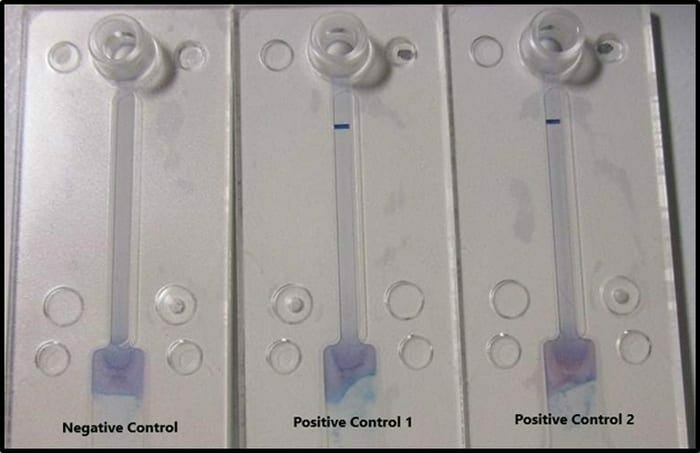
Prototype testing has confirmed successful activation of both the COVID-19 specific probes and the universal coronavirus probes at viral RNA levels that are equivalent to saliva samples from infected human patients.
Negative Control demonstrates no probe activation in the absence of viral RNA.
Positive Control 1 demonstrates activation of the universal coronavirus probes in the presence of non-COVID-19 coronavirus RNA.
Positive Control 2 demonstrates activation of COVID-19 probes in the presence of COVID-19 specific RNA.
 XPhyto is not the only company developing a COVID-19 antigen test.
XPhyto is not the only company developing a COVID-19 antigen test.
Sona Nanotech’s (SONA.C) share price spiked about 25% today (gaining $100 million in market cap) – after announcing that its “rapid detection, COVID-19 antigen technology resulted in a test sensitivity of 96%, test specificity of 96%.”
Sona stated that “sales of the tests will now be permitted under a ‘research use only’ label until full regulatory authority is granted.”
Sona previously predicted that its test will “produce results in 5-15 minutes and is anticipated to cost less than $50”.
XPhyto has not released price targets for a future commercial product.
For reference, home pregnancy tests cost about $1.50 to manufacture and sell for about $15.
Based on 3a’s corporate philosophy, we anticipate that its strategy will be to create a low-cost test for the global market place, including hard-hit countries with strained medical resources like India and Brazil.
3a philosophy:
A1: “anywhere” (no power or additional equipment required)
A2: “anytime” (decentralized and rapidly deployable)
A3: “anyone” (no specialized training required)
“We believe that a low-cost, portable and easy to use screening tool that provides rapid on-the-spot results would be a disruptive tool in the fight against pandemic threats,” stated Hugh Rogers, CEO of XPhyto. “We see an enormous global market opportunity that includes individual households, schools, hospitals, public transportation, airports and border services as well as many private employers.”
Patients infected with an alternate coronavirus strain or a highly mutated form of COVID-19 are expected to activate only the universal coronavirus probes. These patients could be selected for further investigation.
XPhyto’s next developmental milestones:
- Design and manufacture of LFA screening tests
- Initiation of clinical evaluation protocols
- A pilot study using human saliva from healthy and COVID-19 infected patients.
“The global economy could take a hit of some $82 trillion in a worst case scenario from the coronavirus,” according to the University of Cambridge, “In case of speedy recovery, an “optimistic loss” of $3.3 trillion is likely.”
“Testing is the biggest problem that we’re facing,” stated Peter Slavin, president of Massachusetts General Hospital, said recently in a roundtable on Covid-19 at Harvard Medical School.
One month ago, Xphyto announced that 3a and its contract research collaborators, have received a CAD $389,950 grant from the German Federal Ministry of Education and Research (BMBF).
XPhyto’s LFA is designed for use with saliva samples and is also expected to function effectively using test solutions from throat and nasal swabs.
Prototype testing was carried out at 3a’s laboratory in Baden-Württemberg, Germany.
3a expects to begin the pilot study within 30 to 60 days.
– Lukas Kane
Full Disclosure: Xphyto in an Equity Guru marketing client.