Cybin (CYBN.NEO), a Canadian-based biopharmaceutical company working to transform psychedelics into therapeutics, announced today that it had received approval from an independent ethics committee in the Netherlands to commence its first-in-hum dosing of the company’s proprietary deuterated N,N-dimethyltryptamine (DMT) molecule, CYB004.
This marks the first time a deuterated DMT molecule will be evaluated in humans and compresses the time required for Cybin to advance CYB004 to clinic.
The phase 1 CYB004-E trial is being conducted at the Centre for Human Drug Research in the Netherlands and is the largest ever phase 1 DMT trial conducted to date.
DMT is traditionally unstable as a molecule which is rapidly metabolized by the body, which severely inhibits its bioavailability. CYB004 has shown the potential to overcome the limitations of DMT. Using pre-clinical studies, the drug has demonstrated an improved bioavailability and pharmacokinetic profile in comparison with DMT when administered through intravenous (IV) and inhaled routes.
The studies also indicated that CYB004 has a longer duration of effect compared with DMT, revealing a potential to extend the therapeutic window and provide a better dose optimization.
Therefore, CYB004 as a deuterated molecule could very well improve bioavailability compared to DMT, offering a potential of more convenient dosing methods through inhaled, subcutaneous or intramuscular routes of administration.
Doug Drysdale, Cybin CEO, commented, “This is a major milestone for our CYB004 program and for better understanding the potential therapeutic benefits of our proprietary deuterated DMT molecule for the treatment of generalized anxiety disorder. The ability to evaluate our novel CYB004 molecule in humans at this early stage is a significant achievement in clinical development and will provide important insight into the pharmacokinetic and pharmacodynamic properties of CYB004, in addition to what we have already learned through our study of DMT. We expect to apply these findings to optimize dosing and delivery of CYB004 in future clinical trials, which supports our mission to bring this new investigational therapy to patients as quickly as possible.”
Psychedelic therapeutics is an exciting new methodology for treating patients suffering from mental health issues such as depression. The sector is still in its infancy, but advancements in clinical trials such as Cybin’s first-in-human testing continues to push researchers and the industry forward.
The global market for psychedelic therapeutics is estimated to grow to $8.31 billion USD by 2028.
In other news, Cybin announced that it would host a virtual R&D day on Tuesday, February 28, 2023 from 10:00am EST to 11:30am EST.
To register for the event and access the live webcast, you can click here. A Q&A session for the investment community will follow the prepared remarks. Also the archived webcast will be available on Cybin’s investor relations website on the Events & Presentations page following the event.
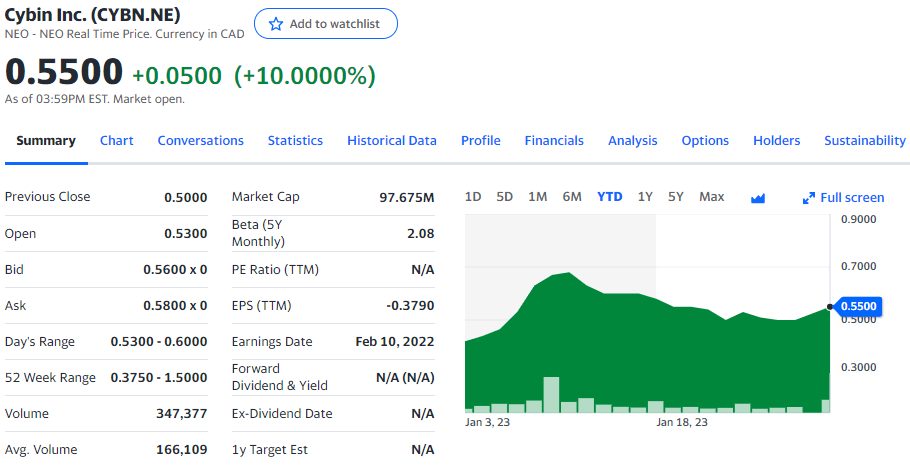
Cybin currently trades at $0.55 CAD per share for a market cap of $97.675 million.