Miraculous Microchip
Yes, you read that right. Earlier this week, German Aldana Zuniga got behind the wheel of an 850 horsepower NASCAR Cup race car. At first glance, this story may not seem incredible. However, Mr. Zuniga is paralyzed from the waist down. He was left paralyzed after a car crash in 2013 that, in addition to leaving him paralyzed below the waist, also limited the use of his arms and hands.
So, how was Mr. Zuniga able to get behind the wheel again? In collaboration with a team led by Scott Falci, M.D., a neurosurgeon at the Falci Institute for Spinal Cord Injuries at Colorado’s Swedish Medical Center, Mr. Zuniga had a microchip implanted in his brain. Mr. Falci is also credited for founding Falci Adaptive Motorsports (FAM) in 2012.
FAM is a nonprofit organization committed to developing adaptive technologies for the mobility-impaired community. Since 2014, FAM has conducted scientific research and development related to cell line development for spinal cord restoration, hands-dree drive technology, and adaptive home systems, among others.
In the same year, Dr. Falci was introduced to Furniture Row Racing (FRR), an American stock car racing team that competed in the NASCAR Cup Series from 2005 to 2018. In collaboration with FRR, Dr. Falci was able to successfully build a safe and controlled adaptive racecar capable of handling fast track use.
How Does it Work?
With a blueprint in hand, Dr. Falci collaborated with Pikes Peak International Raceway to put Mr. Zuniga behind the wheel of their 850 horsepower race car. Using his thoughts, Mr. Zuniga was able to control the throttle while using his helmet to steer. In case of an emergency, he could also engage the breaks by sipping on a straw.
The microchip implanted in Mr. Zuniga’s brain is capable of capturing the “electrical fingerprint” of a thought. In doing so, he is able to control the vehicle’s acceleration and throttle. However, it is important to note that Dr. Falci isn’t trying to create a team of mobility-impaired racecar drivers. Quite the opposite.
Instead, Dr. Falci hopes that this development can be used to drive and control a wide range of technologies, including electric wheelchairs, golf carts, medical devices, or even exoskeleton devices. With this in mind, the microchip created by Dr. Falci and his team is intended to be used in human-machine interface technologies to support functions of daily living, not racecar driving.
TC BioPharm
- $18.938M Market Capitalization
TC BioPharm (TCBP.Q) is a clinical-stage cell therapy company developing allogeneic CAR-T cell therapy products for the treatment of cancer. The Company is focused on the development of gamma delta T-cell therapies for the treatment of infectious diseases. On February 15, 2022, TC BioPharm announced the closing of its Initial Public Offering for aggregate gross proceeds of $17.5 million.
In addition to its gamma delta T-cells, TC BioPharm has also developed cell banks and chimeric co-stimulatory receptor T-cells (Co-Stim CAR-T). The Company’s cell banks are comprised of cellular material collected and stored from healthy donors. These cells are then used to manufacture “next-generation off-the-shelf gamma delta T-cell therapies for future clinical development.”
On the other hand, TC BioPharm’s Co-Stim CAR-T gamma delta T-cells were developed to kill targeted cells without eliminating healthy cells. By sparing healthy cells, the burden of treatment before and after CAR-T treatment can be significantly reduced compared to conventional CAR-T. Keep in mind that conventional CAR-T kills all cells against which they are directed, including normal cells.
Latest News
It is worth noting that gamma delta T-cells are one of three immune cell types that express antigen receptors. In other words, gamma delta T-cells are one of the few T-cells capable of exerting potent cytotoxicity against numerous cancer cells. With this in mind, gamma delta T-cell therapy is regarded as one of the most promising T-cell therapies in clinical development.
We believe that the use of gamma-delta T cell therapy will transform cancer treatment due to the innate ability of these cells to identify and kill cancer cells without targeting healthy cells,” said Bryan Kobel, CEO of TC BioPharm.
On May 23, 2022, Kuick Research announced that it has recognized TC BioPharm as a key player in the gamma delta T-cell therapy market. For context, Kuick Research is a market research and analytics company. In a recent report, Kuick Research estimates that the gamma delta T-cell therapy market will exceed $4 billion by 2028.
In the original report, Kuick Research claims this number is USD$4 million, however, this is definitely a mistake. Moving on, this market is expected to grow as cancer rates continue to rise. Furthermore, the inefficacy of currently available drugs will also accelerate growth in this market.
“We have launched ACHIEVE, a Phase 2b/3 clinical trial for the treatment of acute myeloid leukemia with our allogeneic gamma delta therapeutic OmnImmune® and expect to expand OmnImmune® into additional blood cancer trials in the immediate future,” continued Bryan Kobel.
Our #TCBP Founder and COO, Angela Scott has been asked to join the newly launched UK Government taskforce to boost women starting fast-growing companies! To find out more about this important and exciting initiative, read below! https://t.co/xebiwXcupy #womeninbusiness #ukgov
— TC BioPharm (@TCBioPharm) May 26, 2022
To provide some background, OmmImmune is TC BioPharm’s allogenic unmodified gamma delta T-cell product. OmmImmune is a novel therapeutic targeting the potential treatment of relapsed and refractory Acute Myeloid Leukemia (AML). That being said, the Company’s clinical trial demonstrated that average cancer levels in the bone of patients decreased from 38% to 6%.
It should be noted that OmmImmnune has received Orphan Drug status. Looking forward, TC BioPharm has additional clinical trials planned for 2023 in a number of solid tumor indications. More recently, on May 26, 2022, TC BioPharm announced that its Founder and COO, Angela Scott, has been asked to join the newly launched UK Government taskforce.
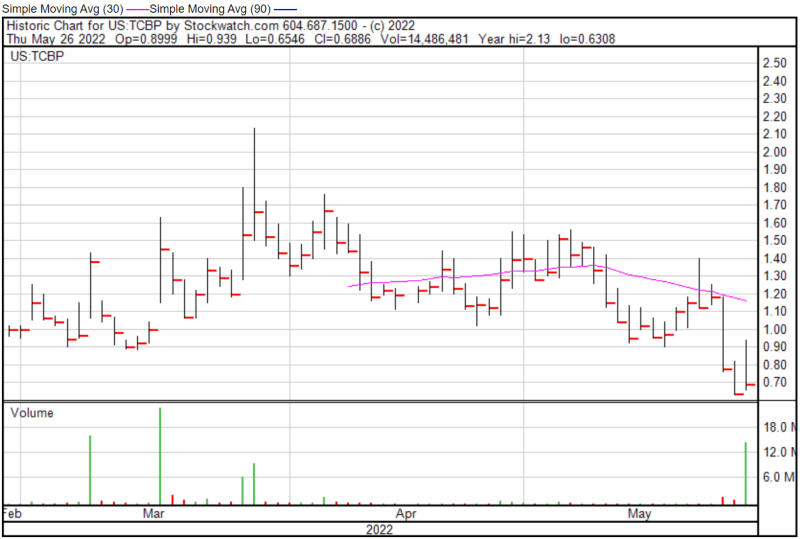
TC BioPharm’s share price opened at $0.8999 on May 26, 2022, up from a previous close of $0.6351. The Company’s shares were up 8% and were trading at $0.6857 as of 11:13 AM EST.
Edesa Biotech Inc.

- $28.141M Market Capitalization
Edesa Biotech Inc. (EDSA.Q) is a clinical-stage biopharmaceutical company focused on developing innovative treatments for inflammatory and immune-related diseases with unmet medical needs. The Company’s product pipeline covers a variety of indications including Acute Respiratory Distress Syndrome (ARDS), Allergic Contact Dermatitis, Hemorrhoids, and Immunotherapy.
Edesa went public on June 10, 2019, via a business combination with Stellar Biotechnologies, a NASDAQ-listed company. Following the combination, Stellar changed its name to Edesa. To date, the Company has completed a total of 5 funding rounds, raising $42.3 million. Edesa’s latest funding round was on March 22, 2022, generating gross proceeds of $10 million.
Edesa’s EB05 is a monoclonal antibody therapy being developed by the Company to treat ARDS, a life-threatening form of respiratory failure and the leading cause of death among COVID-19 patients. According to the US Centers for Disease Control (CDC), 20% to 42% of hospitalized COVID-19 patients develop ARDS. Of those admitted to the ICU, mortality ranges from 39% to 72%.
Latest News
On May 24, 2022, Edesa announced that it has initiated enrollment for the second cohort of patients for the Phase 3 part of a Phase 2/3 study of the Company’s EB05. Previously, Edesa’s first cohort recruited the most critically severe patients receiving mechanical ventilation plus additional organ support, including membrane oxygenation (ECMO) therapy.
“This will allow ICU physicians in the critical care setting to utilize EB05 earlier in the treatment paradigm – before it may be needed as a potential rescue therapy – with the hope of further reducing the number of days in the ICU and preventing more deaths,” said Par Nijhawan, MD, Chief Executive Officer of Edesa Biotech.
According to the World Health Organization’s (WHO) COVID-19 Severity Scale, these patients classify as Level 7. With this in mind, Edesa’s second cohort is open to hospitalized patients on invasive mechanical ventilation, otherwise referred to as Level 6 patients, according to WHO. As for the study’s primary endpoint, the Company will use the number of ventilation-free days for Level 6 patients.
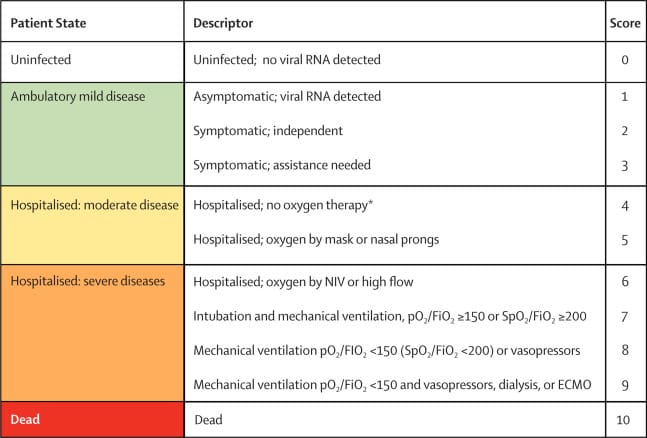
To be more specific, Edesa will record the number of ventilation-free days on Day 28 following administration of a single intravenous infusion of EB05. Secondary endpoints will include ventilation-free days on Day 60 as well as the mortality rate on Day 28 and Day 60. Protocol for the second cohort of Level 6 patients requires approximately 500 evaluable subjects.
Bearing this in mind, the primary and secondary endpoints of Edesa’s second cohort are almost identical to the first cohort. However, the primary endpoint of the Company’s first cohort of Level 7 patients will include the 28-mortality rate. Moreover, the first cohort calls for roughly 315 subjects, compared to 500. Edesa is currently in discussions in the US regarding the approval of the final Phase 3 design.
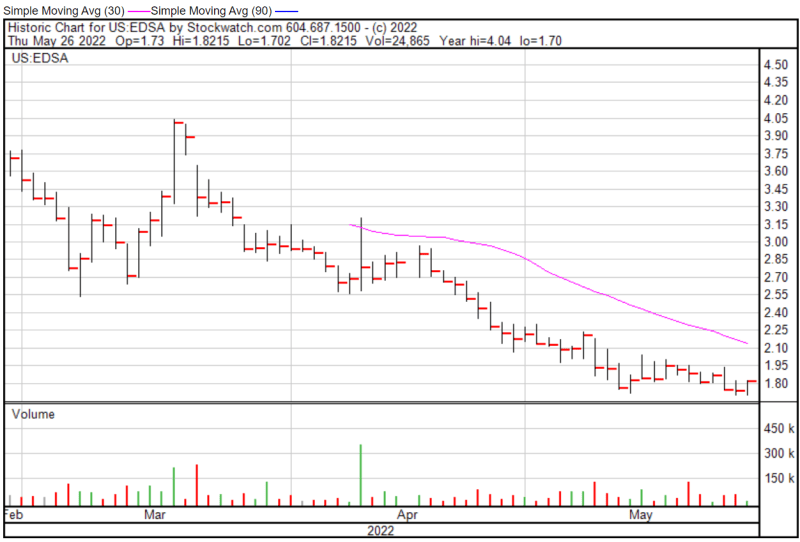
Edesa’s share price opened at $1.73 on May 26, 2022, up from a previous close of $1.72. The Company’s shares were up 5.90% and were trading at $1.8215 as of 11:56 AM EST.
Bionano Genomics Inc.

- $453.376M Market Capitalization
Bionano Genomics Inc. (BNGO.Q) is a genomics company, credited for its Saphyr System. For context, genomics refers to an interdisciplinary field of biology focusing on the structure, function, evolution, mapping, and editing of genomes. The Company went public on August 21, 2018, with an Initial Public Offering of 3.36 million units at $6.125 each. Following its IPO, Bionano was able to generate total gross proceeds of $20.58 million.
Bionano’s Saphyr System is an Optical Genome Mapping (OGM) platform intended to identify structural variations (SV). This enables scientists to isolate DNA and identify different codes as well as SVs. Keep in mind that some SVs are responsible for genetic diseases such as autism. Bionano’s Saphyr System is intended to identify these SVs in one streamlined workflow, compared to the overcomplicated process of traditional genome analysis methods.
Latest News

On May 26, 2022, Bionano announced the publication of the first peer-reviewed research study using OGM to analyze genetic aberrations found in pediatric patients with AML. This research demonstrates the use of OGM to identify minimal residual disease (MRD) markers and other novel variants. For context, MDR refers to the small number of cancer cells that remain in the body after treatment.
“Oncologists are often left with no genetic markers to follow during treatment. OGM performed well compared to traditional methods, but the compelling value proposition is its ability to add incremental findings that have the potential to impact disease monitoring and outcomes,” said Erik Holmlin, Ph.D., President, and chief executive officer of Bionano Genomics.
So why is this important? The detection of MRD can be followed over the course of treatment. In doing so, MDR can be used to determine whether treatment is working or if a patient is relapsing. During this study, 70% of cases revealed significantly more SVs when using OGM compared to traditional cytogenetic methods.
In particular, 32 previously unknown aberrations were detected, with one high-risk marker detected by only OGM. Additionally, in two cases, OGM detected novel fusion partners of a gene known to play a role in hematopoiesis, which refers to the formation of blood in the body.
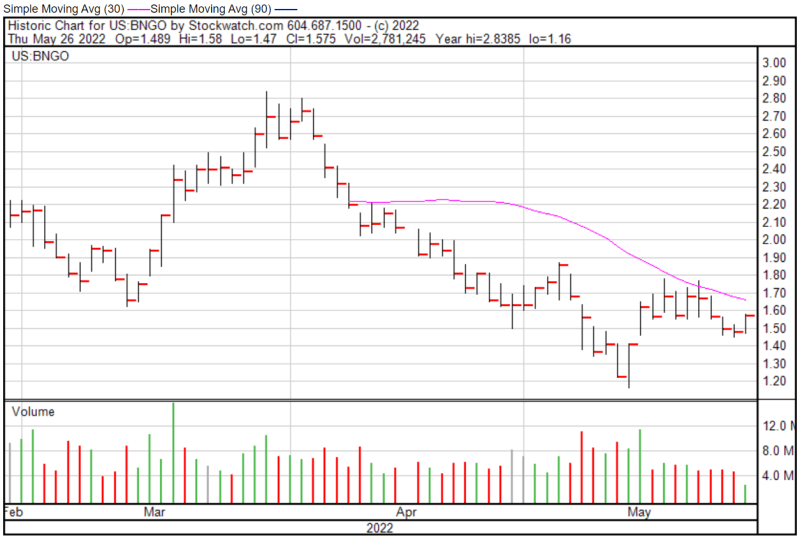
Bionano’s share price opened at $1.51 on May 26, 2022, up from a previous close of $1.48. The Company’s shares were up 5.74% and were trading at $1.565 as of 12:52 PM EST.
Ocugen Inc.

- $483.083M Market Capitalization
Ocugen Inc. (OCGN.Q) is a biotechnology company focused on the discovery, development, and commercialization of novel gene therapies, biologicals, and vaccines. Ocugen completed its initial public offering for gross proceeds of $65,000 on December 8, 2014. However, the Company began trading on the NASDAQ on December 3, 2014.
Regarding Ocugen’s vaccines, the Company has developed its COVID-19 vaccine candidate BBV152, also known as COVAXIN™ outside of the US. BBV152 has demonstrated a vaccine efficacy in mild, moderate, and severe COVID-19 cases of 77.8% with efficacy against severe COVID-19 cases of 93.4%. BBV152 has also been granted Emergency Use Listing by the WHO.
Latest News
Now, let’s talk about Ocugen’s modifier gene therapy platform, comprised of OCU400, OCU410, and now NeoCart®. On May 24, 2022, Ocugen announced NeoCart, a Phase 3 cell therapy platform. NeoCart was recently granted a Regenerative Medicine Advanced Therapy (RMAT) designation for the repair of full-thickness lesions of the knee cartilage in adults.
“Our next step will be working with the FDA to construct the Phase 3 program to bring this innovation to this emerging treatment area. We believe that NeoCart® offers the potential for an innovative new option where treatments in this area are still limited and results are not optimal,” said Dr. Shankar Musunuri, Chairman, CEO, and Co-Founder, Ocugen, Inc.
To provide some context, an RMAT designation was created to expedite the development and review of regenerative medicine therapies. An RMAT designation carries with it all of the benefits of fast track and breakthrough therapy designation programs. This includes early interactions with the FDA. With this in mind, Ocugen is currently in discussions with the FDA to finalize the Phase 3 protocols needed to advance the clinical development of NeoCart.
Ocugen’s share price opened at $2.15 on May 26, 2022, down from a previous close of $2.17. The Company’s shares were up 3.69% and were trading at $2.25 as of 1:22 PM EST.