Edesa Biotech (EDSA.Q) announced positive results today from its Phase 2 portion of an ongoing Phase 2/3 clinical study. This study is intended to evaluate EB05, the Company’s monoclonal antibody candidate, as a single-dose treatment for hospitalized COVID-19 patients.
“The strong effect in reducing death in the most critically ill hospitalized patients who have been treated with systemic corticosteroids, including dexamethasone, and IL-6 inhibitors, shows the potential life-saving impact of this drug, irrespective of SARS-CoV-2 variant,” said Dr. Par Nijhawan, MD, Chief Executive Officer of Edesa Biotech.
Edesa is a clinical-stage biopharmaceutical company focused on developing innovative treatments for inflammatory and immune-related diseases with unmet medical needs. With this in mind, I can’t say I am too surprised that a company like Edesa has undertaken the development of a treatment for SARS-CoV-2. Normally, I would chalk this up as just another COVID-19 press release from a generic biopharmaceutical company, however, Edesa announced some impressive results in its press release today. Before we get into that, let’s talk about the Company’s EB05. EB05 is a monoclonal antibody therapy being developed by Edesa to treat Acute Respiratory Distress Syndrome (ARDS), a life-threatening form of respiratory failure and the leading cause of death among COVID-19 patients. According to the U.S. Centers for Disease Control (CDC), 20% to 42% of hospitalized COVID-19 patients develop ARDS, which increases up to 85% for patients admitted to the ICU. Overall, mortality among patients admitted to the ICU ranges from 39% to 72%.
As previously mentioned, Edesa is currently investigating EB05 as a treatment for ARDS in hospitalized COVID-19 patients through a Phase 2/3 clinical study. It is worth noting that Edesa’s study is being funded in part by a CAD$14 million grant from the Canadian Government. Not bad. So, just how impressive are Edesa’s Phase 2 results? For starters, an independent Data and Safety Monitoring Board (DSMB) informed Edesa that during its initial analysis, it identified a clinically important efficacy signal and concluded that the study has met its objective. Furthermore, the DSMB requested that the study be preemptively unblinded. For context, a DSMB is an independent group of experts responsible for evaluating a study’s safety, study conduct, and scientific validity. With this in mind, for the DSMB to request an unblinding, the results must have been pretty damn good.
The Results
Now, for the moment you have all been waiting for. Among the findings, the DSMB reported a 28-day death rate of 14.3% in the EB05 arm versus 36.8% in the placebo arm in critically severe patients receiving extracorporeal membrane oxygenation (ECMO) therapy. In layman’s terms, ECMO refers to life support, where cardiac and respiratory functions are prolonged and supported. With this in mind, survival analysis indicated that patients treated with EB05 in combination with standard of care had a whopping 68.5% reduction in the risk of dying when compared to placebo. Moreover, results from the Phase 2 analysis also suggest that EB05 has been generally well-tolerated and consistent with the observed safety profile to date. Additionally, the DSMB identified another patient group with robust signals for morality reduction at 28 days.
Looking forward, Edesa plans to review this data and will focus on patient segments that have demonstrated the strongest efficacy signals. The Company also intends to file amendments with regulators in the U.S., Canada, and Colombia to update the Phase 3 protocol and set targeted enrollment. Lastly, Edesa is evaluating opportunities to apply for expedited regulatory review programs in the U.S. and Canada. If you’re interested in other biopharmaceutical companies combating COVID-19, check out a few of the companies we have covered on our site.
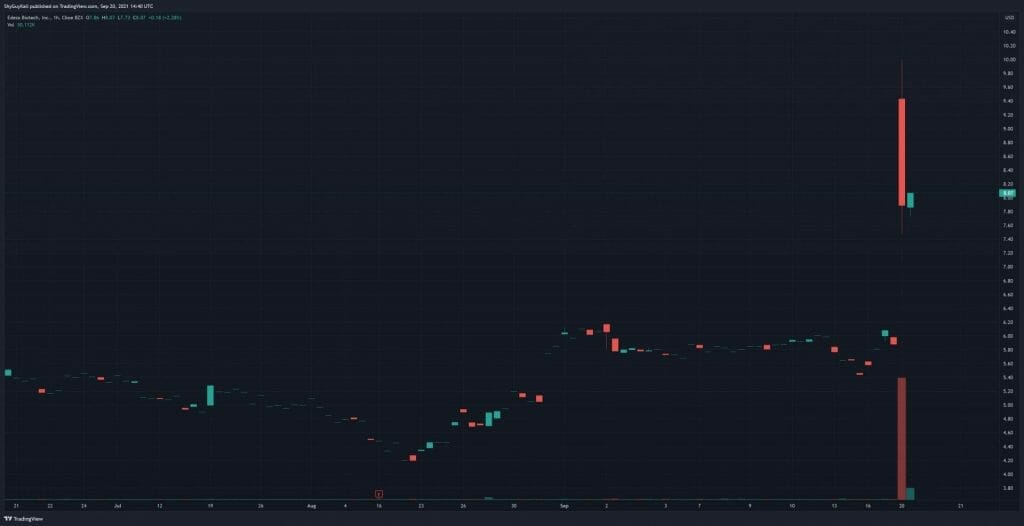
Edesa’s share price opened at $9.45, up from a previous close of $5.88. The Company’s shares are up 39% and are currently trading at $8.18 as of 10:42 AM ET.