Tetra Bio-Pharma (TBP.T) announced today that the Central Ethics Committee has approved the proposed protocol modifications allowing additional immediate release oral opioids to be used as a comparator in the REBORN1© study. Expanding to three immediate release oral opioids will allow for the acceleration of patient enrolment in the trial.
“The amended protocol allows QIXLEEF™ to be studied against three types of immediate release oral opioids instead of just one. It will facilitate the recruitment of patients who can benefit from QIXLEEF™ to treat their breakthrough pain as it broadens the pool of qualified patients. Once the Schedule I license is approved, a process that usually takes a couple of weeks, the amended protocol will enhance the recruitment rate, thereby accelerating the study conduct…,” said Dr. Guy Chamberland, CEO and CRO of Tetra.
Opioids come in a variety of forms including heroin, fentanyl and even prescription pain relievers. Typically prescribed for issues like chronic pain, opioids have become the standard for pain alleviation and management, however, roughly 21-29% of patients who are prescribed opioids for chronic pain misuse them. An example of prescription opioid misuse includes crushing or opening a capsule, dissolving the contents in water, and injecting the liquid into one’s veins. As a result of misuse, nearly 50,000 people in the United States died from opioid related overdoses in 2019.
With this in mind, Tetra Bio-Pharma is committed to combating the ongoing opioid crisis that exists not only in the United States, but around the world. The REBORN1 trial is a head-to-head Phase 2 study against opioid treatment in the management of short and frequent episodes of breakthrough pain in patients living with cancer. For context, breakthrough pain refers to severe or excruciating pain that can disable or immobilize a patient. The initial protocol for REBORN1 was to compare the safety and efficacy of QIXLEEF™ to conventional oral morphine sulfate immediate release, a common opioid used for pain management.
What is QIXLEEF?
QIXLEEF is Tetra’s inhaled proprietary drug formulation containing a fixed ratio of THC and CBD. The Company’s drug formulation is intended to provide a safer alternative to common prescription pain management opioids like morphine sulfate immediate release.
“The approval by the DEA is a formality as the amendment does not modify the amount of controlled substance at the clinical site. Further, there have been numerous news reports recently of drug makers exiting the opioid business, however for patients suffering from uncontrolled pain, this news does not provide them with an alternative to alleviate their pain. Tetra is in the business of providing the evidence which would see QIXLEEF™ become an alternative prescription therapy to opioids,” continued Mr. Chamberland.
The approved amended protocol will allow the recruitment of cancer patients living with breakthrough pain who are treated with either morphine sulfate immediate release, oral hydromorphone immediate release, or oral oxycodone immediate release. By facing off against three immediate relief oral opioids, Tetra now has the opportunity to accelerate patient enrollment and demonstrate QIXLEEF’s potential as an alternative for pain management.
Although this is excellent news for Tetra, the Company’s financials aren’t looking as promising. According to Tetra’s Q1 2021 financial statement, the Company’s total assets have been reduced to $28,194,788 from $29,338,545 in the previous year. On the other hand, the Company’s total liabilities have increased from $6,749,759 to $12,034,917 in the same period. Overall, Tetra’s cash for the three months ended February 28, 2021 was down to $1,028,487 from $11,971,936 year-over-year. However, the commercialization of Tetra’s pipeline of products, including QIXLEEF, could strengthen the Company’s financials significantly.
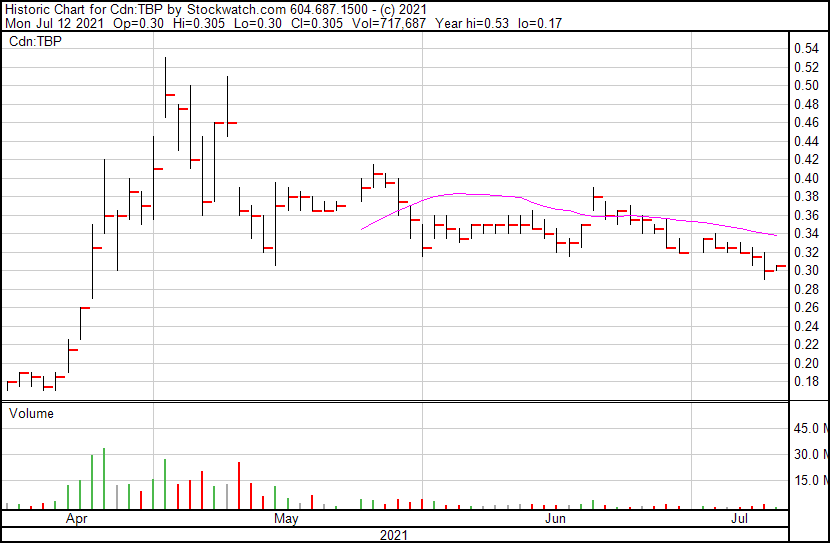
Tetra’s share price opened at $0.30. the Company’s shares are up 1.67% and are currently trading at $0.305 as of 10:55AM ET. This indicates that there has been some change following the news.