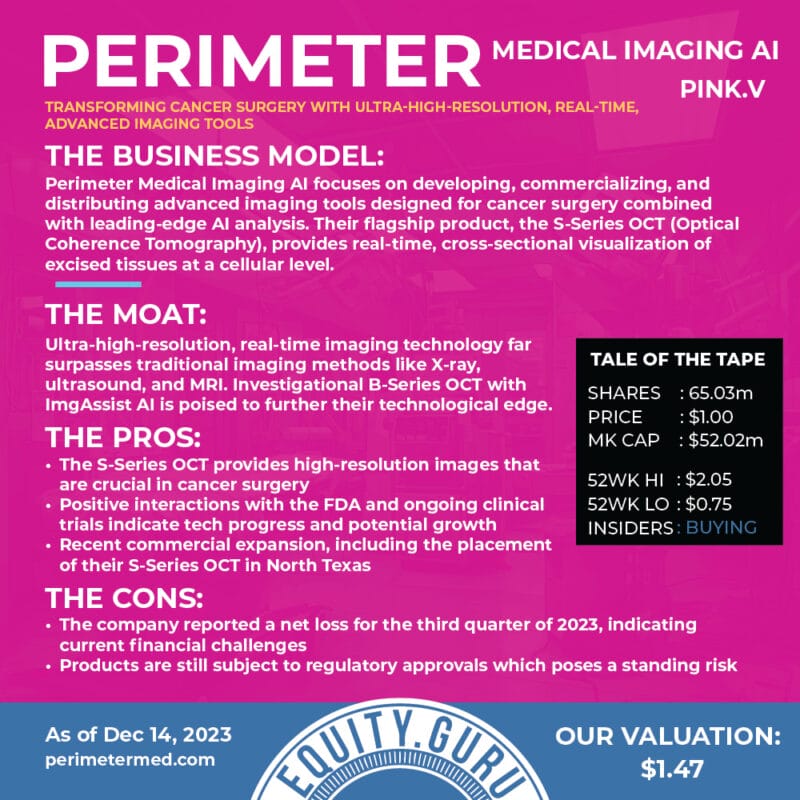
In the biotech/medtech world, the steps to viability as a company are proving your technology works, getting that technology approved by the FDA, finding doctors who are interested in using it, and commercializing the product so it can be manufactured to scale.
Perimeter Medical Imaging (PINK.V) just made it through the final boss level.
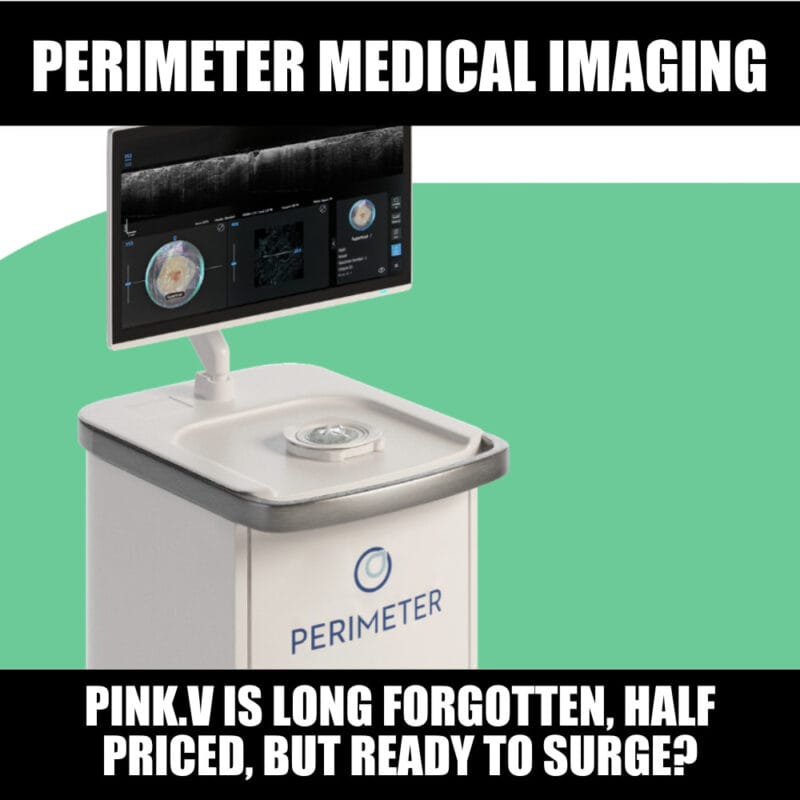
Perimeter Medical Imaging AI Inc. has successfully completed the technology transfer of its manufacturing process for the production of its optical coherence tomography (OCT) imaging systems with Minnetronix Medical, a leading medical technology and operations partner to global medical device companies. [..] “For the first time, the Perimeter S-Series OCT was manufactured at commercial scale, and we believe this collaboration results in a highly scalable, fast and efficient commercial manufacturing process that enables us to meet our customers’ needs in a capital-efficient manner,” [said Perimeter CEO Jeremy Sobotta.]
In the overall scheme of things, this is a monumental milestone. It drives down the cost to manufacture, speeds up the time to manufacture, and allows the company to focus on sales to the end user.
Generally, building a medical technology company comes with a variety of potential breaking points that can scupper, or at least slow down, plans for commercialization. The FDA approval process can be a labyrinthian ordeal like something out of the movie Brazil, as can securing the appropriate patents, or even just keeping cash in the bank over the years of trials and testing.
But taking the technology to a finished product, completed by a reliable medical tech manufacturer, can be its own nest of vipers.
That PINK has surged into commercialization is a massive vote of confidence for what this team has built.
Mr. Sobotta continued: “The partnership with Minnetronix Medical provides us access to cutting-edge manufacturing techniques and the scale of a leading medical device manufacturer. This, combined with their expertise and focus on optical systems, makes them an ideal partner as we execute on our commercial plans going forward.”
Having access to a strong supplier base is only one of the advantages of partnering with a recognized medical manufacturer. With state-of-the-art production options, the end product will add a selling point to any doctor or hospital that looks to add the product to its facilities.
The medical system is in desperate need of this tool.
Cleared by the U.S. Food and Drug Administration, Perimeter S-Series OCT is a novel medical imaging system that provides clinicians with cross-sectional, real-time margin visualization (one millimetre to two millimetres below the surface) of an excised tissue specimen. Giving physicians the ability to visualize microscopic tissue structures in real time in the operating room has the potential to result in better long-term outcomes for patients and lower costs to the health care system.
In other words, when a surgeon is is removing potentially cancerous tissue, they can see during the process whether they’ve reached everything needed, without having to rely on tissue specimen testing that can take weeks. This means less need for follow-up surgeries and better success rates.
The FDA granted breakthrough device designation for Perimeter B-Series OCT coupled with ImgAssist AI, and Perimeter has plans to initiate a randomized, multisite, pivotal study to evaluate it against the current standard of care and assess the impact on reoperation rates for patients undergoing breast conservation surgery.
I’ve been invested in this company for some time, and not only has it been profitable for me as an investment, but I feel all the better knowing my dollars are helping it exist in the world.
— Chris Parry
FULL DISCLOSURE: Perimeter Medical Imaging is an Equity.Guru marketing client and we own stock in the company.