Cybin (CYBN.NE) announced that the Institutional Review Board (IRB) has granted them approval to begin the study of its sublingual psilocybin formulation, CYB001, in a Phase II clinical trial for patients suffering from Major Depressive Disorder (MDD).
The study is taking place at the University of West Indies Hospital in Jamaica, and the commencement of the study is subject to final approval of study material specification by Jamaica’s Ministry of Health.
The Phase IIa study will involve 40 patients and will aim to identify the bio-equivalent dose of Cybin’s proprietary sublingual psilocybin formulation versus a 25mg oral capsule. Instead of swallowing a capsule, CYB001 is placed under the tongue which enables rapid absorption of the molecules into the bloodstream, avoiding first-pass metabolism of the substance. This feature allows faster onset, shorter duration, and a lower effective dose.
The press release also stated that, upon successful completion of the Phase IIa study, Cybin will proceed to a Phase IIb clinical study. The Phase IIb study will be a randomized, placebo-controlled study with 120 patients with MDD. The efficacy of CYB001 will be measured by observing any reductions or increases in patients’ scores on the Montgomery-Asberg Depression Rating Scale (MADRS) at 30 days.
Cybin also said their clinical trial will adhere to ICH-GCP guidelines, allowing them to use the clinical data across the world, including in Canada, the US, and Europe.
“IRB approval for our study protocols is an important step forward to begin testing our proprietary psilocybin formulation delivered via absorption under the tongue in patients with Major Depressive Disorder. We are planning several additional studies to expand our clinical understanding of this potentially ground-breaking therapeutic. Cybin continues to expand on its 4 active drug programs targeting depression, addiction and other psychiatric conditions alongside its growing portfolio of 50+ proprietary psychedelic molecules. This latest IRB approval moves Cybin closer to unlocking the potential of more scalable therapeutics,” stated Doug Drysdale, Chief Executive Officer of Cybin.
Sorry, did you say earlier that these studies are taking place in Jamaica?
Yes, and thank you imaginary reader for your useful question.
As my Equity Guru colleague Taylor Gavinchuk noted back in March, many companies are setting up shop in Jamaica to take advantage of their more relaxed laws around psilocybin use. Companies like Field Trip (FTRP.C) have set up research centers on the island, while others, like Psyence Group, have taken a “research meets retreat” approach. As they are partnering with a university hospital, Cybin is opting for the former approach. This will allow them to conduct research they can later use for things like FDA applications.
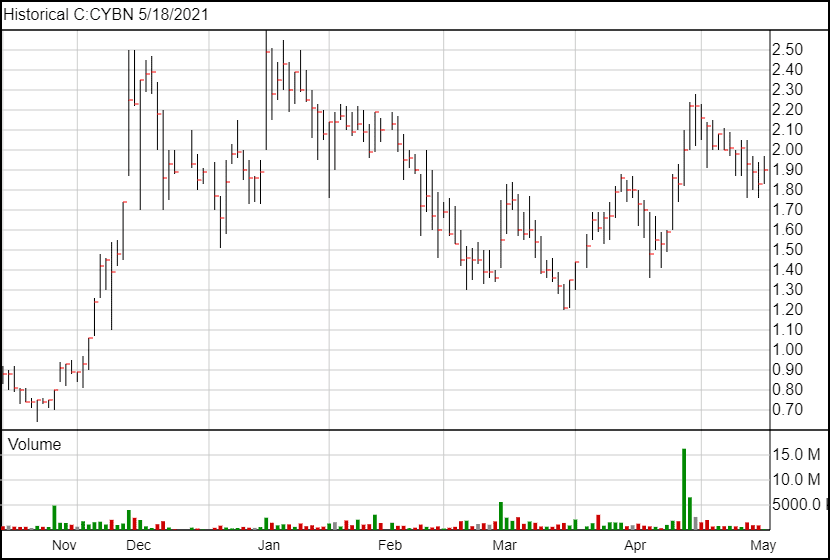
Following the news, CYBN shares are up seven cents and are currently trading at $1.90.
Full disclosure: Cybin is an Equity Guru marketing client.