Tetra Bio-Pharma (TBP.T), a leader in cannabinoid-derived drug discovery and development, announced today that it has been granted a Health Canada Drug Establishment License (DEL) to distribute REDUVO soft gel capsules in Canada.
“We look forward to working closely with our Contract Manufacturing Organization for the importation, warehousing, and dissemination of the THC pharmaceutical drug to pharmacies across Canada. We stand by our commitment to launch our THC-based prescription drug to the Canadian market in late 2021,” commented Tetra’s CEO, Dr. Guy Chamberland.
REDUVO is a synthetic THC-based soft gel capsule indicated in severe nausea and vomiting associated with cancer chemotherapy. In the United States, REDUVO is known as Marinol and has been approved as a regulated pharmaceutical drug by the U.S. Food and Drug Administration (FDA) since 1985. Marinol and REDUVO both contain dronabinol, a specific form of tetrahydrocannabinol (THC) used to treat chemotherapy-induced vomiting as well as HIV/AIDS-induced anorexia.
According to Grand View Research, the global cannabis pharmaceuticals market is expected to grow at a compound annual growth rate of 76.8% from 2020 to 2027. This market includes products like REDUVO and Marinol. With this in mind, the global cannabis pharmaceuticals market is still in its infancy, but it is expected to grow rapidly as cannabis becomes increasing normalized in the medical sector.
On April 1, 2021, Tetra Bio-Pharma announced that it had received a compliant rated Good Manufacturing Practices (GMP) inspection by Health Canada. The GMP system ensures that manufacturing products, such as food, cosmetics, and pharmaceutical goods, are consistently produced and controlled according to set quality standards. Having met strict quality control standards and procedures, Health Canada has granted the Company with a DEL.
What’s the big deal? Well, any company in Canada looking to distribute pharmaceutical drugs must obtain a DEL from Health Canada. Having received its DEL, one of the most daunting licenses to earn, Tetra Bio-Pharma has achieved a huge milestone towards the commercialization of REDUVO in Canada and plans to launch the THC-based prescription drug in the Canadian market in late 2021. Currently, REDUVO is being assessed by Health Canada for the issuance of a Drug Identification Number (DIN), which indicates that the product has undergone and passed a review of its formulation, labeling and instruction for use. Additionally, Tetra Bio-Pharma has applied for its Canada Cannabis Drug License (CDL), a necessary license for companies that intend to distribute pharmaceutical drugs containing cannabis in Canada.
That’s a lot of hurdles to jump over, however, Tetra Bio-Pharma is now inches away from releasing REDUVO in Canada’s pharmaceutical market. In addition to REDUVO, the Company recently shipped Qixleef, its investigational drug, to the U.S. as a potential treatment for breakthrough pain. With this in mind, keep an eye out for information pertaining to the Company’s Qixleef study and the upcoming launch of REDUVO in Canada. 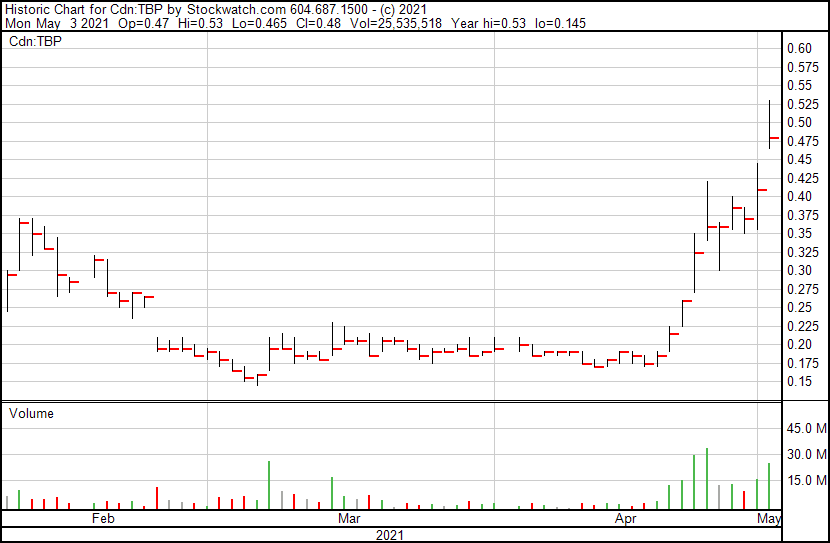
Tetra Bio-Pharma’s share price opened at $0.47, up from a previous close of $0.41. The Company’s shares are up 17.07% and currently trade at $0.48 as of 3:18PM ET. This indicates that there has been some notable change following the news.