On October 04, 2018 Vivo Cannabis (VIVO.V) announced that it has entered into an agreement with Pharmascience which will make it easier for patients to use medical cannabis.
Pharmascience is a global pharmaceutical company based in Montreal that holds a Dealers License from Health Canada.
On behalf of Vivo, Pharmascience will employ “pharmaceutical quality standards” to create cannabis formulations that maximize therapeutic benefit to patients.
Vivo believes that health care professionals and patients will welcome the availability of precisely-controlled, high-quality, standardized dosage forms of cannabis.
“We are pleased to combine our strengths with those of Pharmascience for the benefit of our current and future medical patients,” stated Barry Fishman, CEO of Vivo.
Pharmascience is not small potatoes. This 35-year-old private company has 1,500 employees and product distribution in over 60 countries.
Pharmascience manufactures and markets generic, over-the-counter, and behind-the-counter products, as well as FDA approved Canadian-made injectables – covering 300 product families in 20 different dosage forms.
In Canada alone, about 45 million prescriptions a year are filled with Pharmascience products.
This collaboration strengthens Vivo’s ability to penetrate the Canadian medical marijuana market – estimated to be worth $2 billion by 2020.
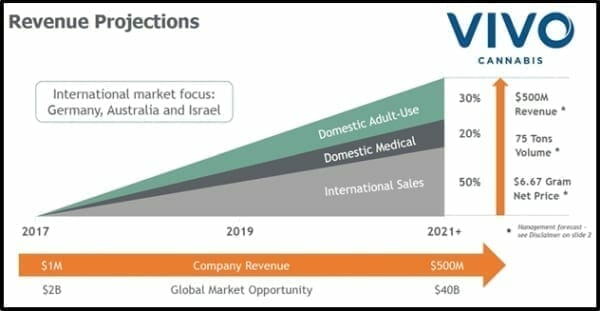
The image below depicts a birds-eye view of Vivo’s macro ambitions.
On October 03, 2018 Vivo announced that it made a $5 million investment in Westleaf Cannabis.
Westleaf’s mission is to create customer engagement, through music and cannabis culture. The company is constructing cannabis production facilities in Alberta and Saskatchewan.
“This investment by Vivo and the associated supply agreement are a strong endorsement of our strategy,” stated Scott Hurd, President and CEO of Westleaf, “Vivo’s reputation for delivering high-quality, trusted and innovative cannabis products that resonate with consumers, aligns well with Westleaf’s innovative retail concept and strategy.”
“Vivo will continue to pursue opportunities across the full value chain,” stated Fishman, “We look forward to working with the Westleaf team to bring our premium products to market.”
Key Westleaf Deal Points:
- Multi-year supply agreement
- Vivo supplies cannabis to Westleaf.
- Vivo gains access to multiple retail locations
- Westleaf absorbs VIVO’s portfolio of adult-use brands
- FIRESIDE, Lumina and Canna Farms products delivered to Westleaf
- Westleaf’s retail footprint is subject to regulatory approvals
Last week Vivo signed a deal with national cannabis distributor, Green Hedge Education & Distribution (GHED).
“The multi-year deal will facilitate the supply of Vivo Cannabis’ product to licensed retailers and wholesalers nationwide,” wrote Equity Guru’s Ethan Reyes.
Green Hedge is a “results-driven recreational cannabis distribution team” to representing Vivo’s key brands, including FIRESIDE, Lumina and Canna Farms.
On September 5, 2018 Vivo completed the acquisition of Canna Farms, British Columbia’s first licensed grower.
The acquisition cost Vivo $133 million, consisting of $22.5 million in cash and the issuance of 92.5 million common shares priced at $1.20 each.
Key Canna Farm Deal Points:
- Enhanced Financial and Capital Markets Profile: strong cash position of $100 million.
- Canna Farms’ positive operating cash flow – $9.4 million revenue EBITDA
- Increased Capacity and Scale: Production capacity of 57,000 kilograms.
- Multiple provincial supply agreements already secured.
- International Leverage: Expedited expansion strategy into international markets, with a focus on Germany and Australia.
On September 24, 2018 Vivo announced the launch of the new Beacon Medical website – which will help patients, doctors, and caregivers “navigate the complex medical cannabis market.”
“Beacon Medical aims to provide a clear path to understanding medical cannabis,” stated Sung Kang, Vivo Chief Marketing Officer in the press release.
The rebuilt website features expanded discount programs, a new classification system called Beacon Cannatypes and “simple categories that help consumers select from the hundreds of strains of cannabis available in Canada”.
VIVO also operates Harvest Medicine, “a patient-centric and highly scalable network of specialty medical cannabis clinics as well as a new free telemedicine app that provides best-in-class education and support to over 15,000 patients.”
Two weeks from now, weed will be legal in Canada. The domestic market is estimated to be worth between $4 billion to $12 billion annually.
We’ve written a lot about branding as it pertains to the cannabis space, and the challenges that will face any licensed producer as it transitions from growing to marketing to retail.
“Those challenges are plentiful,” writes Equity Guru’s Chris Parry, “chiefly among them, you’re not allowed to market your weed brand in Canada.”
The medical market can be accessed directly through partnerships with pharmaceutical companies.
“We are very excited to associate with VIVO, a recognized Canadian Licensed Producer with a strong focus on quality and innovation,” stated David Goodman, CEO of Pharmascience.
Full Disclosure: Vivo is an Equity Guru marketing client, and we own stock.



Question regarding the box statement at the end of this article , i.e.
“The medical market can be accessed directly through partnerships with pharmaceutical companies.”
This implies
A. That marketing/promoting for specific medical products (in this case Vivo) is done by pharmaceutical companies (in this case Pharmascience). How is this done?
Does Pharmascience ask Doctors or clinics to promote their catalog of products (i.e. Vivo products) to medical marijuana patients?
B. That “you’re not allowed to market your weed brand in Canada” is sort of bypassed
by pharma companies through direct access to medical market patients. (Is this right or am
I wrong on this conclusion?)
Dear Ian, your questions deserved good answers, so I had a chat with Vivo, and they told me this: “VIVO has a development agreement with Pharmascience and don’t currently distribute any VIVO products. There are very specific rules and regulations around how companies market their products in both the medical and rec markets and VIVO endeavors to comply with all of these.” Decoding this a bit, I believe the marketing strategies you referred to are in flux. I suspect in time the definitive answers will come from Pharmascience. I regret the lack of clarity. Maybe that is “the take-away.” There is a lack of clarity.