Special Delivery

- $19.043M Market Capitalization
Lexaria Bioscience Corp. (LEXX.Q) is a biotechnology company developing DehydraTECH™, the Company’s proprietary drug delivery technology. In addition to enhancing the speed and efficiency of orally-delivered drugs, DehydraTECH has demonstrated effectiveness in improving delivery through human skin for the potential development of topically-administered products such as patches.
Through various animal studies evaluating the quantity of drug delivery across the blood-brain-barrier (BBB) utilizing DehydraTECH technology, data suggests a gain of as much as 1,900%. With this in mind, Lexaria’s DehydraTECH has the potential to improve the delivery of certain central nervous system (CNS) targeted drugs against Alzheimer’s Disease, Parkinson’s Disease, and other CNS diseases.
In addition to CNS diseases, Lexaria’s DehydraTECH has been granted patents for antiviral drugs, cannabinoids, nicotine, non-steroidal anti-inflammatory drugs (NSAIDs), and vitamins. The Company also has a few patents pending, including DehydraTECH for phosphodiesterase inhibitors, estrogen, and testosterone. So how does Lexaria’s posterchild DehydraTECH actually work?
The Science. The Results.
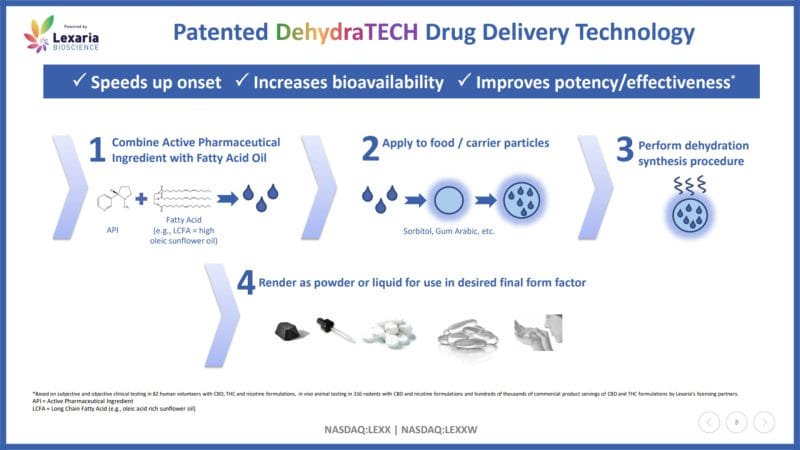
According to Lexaria, DehydraTECH combines long-chain fatty acids (LCFAs) with active pharmaceutical ingredients (APIs). When orally ingested, these DehydraTECH-enabled APIs enter the upper intestine just minutes after administration. These drugs are then primarily transported into the lymphatic lacteals, which are responsible for absorbing digested fats, instead of the liver.
As a result, DehydraTECH-enabled drugs are able to directly enter blood circulation without being metabolized by the liver. This allows drugs to maintain their native form, travel through the bloodstream, and reach the receptor cells in the brain. With this in mind, Lexaria’s drug delivery technology boasts rapid onset, increased bioavailability, and potency.
In total, the global drug delivery market was valued at USD$39.33 billion in 2022. This market is expected to reach USD$71.75 billion by 2029, expanding at a compound annual growth rate (CAGR) of 9%. It should be noted that the onset of the COVID-19 pandemic accelerated the growth of the drug delivery market relative to increased vaccine development.
In fact, the global drug delivery market experienced 26.2% growth in 2021 compared to the year prior. Bearing this in mind, Lexaria’s DehydraTECH has demonstrated enhanced oral delivery of antiviral drugs into animal bloodstreams. These drugs include darunavir, efavirenz, remdesivir, ebastine, and colchicine.
In particular, DehydraTECH enabled remdesivir and ebastine showed effectiveness in inhibiting the SARS-CoV-2 virus in an animal study. On June 3, 2021, Lexaria announced preliminary results from its VIRAL-C21-3 study. The VIRAL-C21-3 study successfully verified that remdesivir and ebastine did not lose their efficacy when processed with DehydraTECH.
High Demand
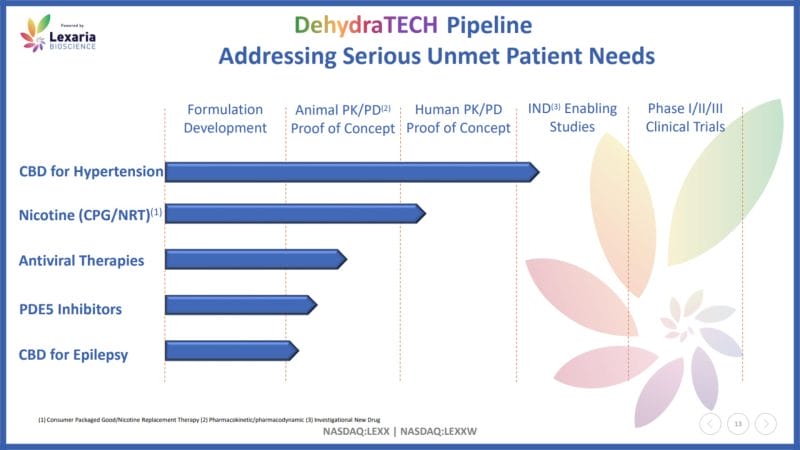
On October 31, 2017, Lexaria announced that it had received a Notice of Allowance from the US Patent and Trademark Office (USPTO) for the use of DehydraTECH for all cannabinoids, including THC, fat-soluble vitamins, NSAIDs, and nicotine. With this in mind, the Company’s DehydraTECH shares a deep development history with cannabis. Since then, Lexaria has developed numerous CBD-based programs.
For example, on May 6, 2021, Lexaria announced results from its HYPER-A21-1 hypertension study. For context, hypertension, also referred to as high blood pressure, is a common condition where pressure against the blood vessel walls is consistently high. Results from Lexaria’s first hypertension study demonstrated up to 2,178% more CBD in the bloodstream of ten male Sprague-Dawley rats.
The study also revealed up to 1,737% more CBD was delivered into the brain tissue. Shortly after, on May 20, 2021, Lexaria announced that DehydraTECH 2.0, the Company’s newest CBD formulation, delivered up to 2,708% more CBD into the bloodstream, representing the strongest absorption achieved by DehydraTECH to date.
In addition to animal studies, Lexaria has and continues to conduct a variety of human clinical trials utilizing DehydraTECH for arterial stiffness and pulmonary hypertension. With this in mind, the Company’s technology is both versatile and effective. As a result, Lexaria has caught the attention of several companies spanning a variety of industries.
Licensing Agreements
In the last month, Lexaria has established numerous licensing agreements. On June 2, 2022, the Company announced the awarding of a European and United Kingdom (UK) DehydraTECH license for medical cannabis applications to Valcon Medical A/S. Valcon specializes in manufacturing medical cannabis extracts for the European Union (EU) and the UK.
The company’s registered medical cannabis products include bulk powders, powder-filled capsules, compressed tablets, pills, oral melts, and topical creams. Lexaria’s non-exclusive licensing agreement with Valcon includes a combination of defined milestone fees and royalties payable to Lexaria. This agreement demonstrates progress toward the Company’s goal of expanding in the EU.
Shortly after, on June 3, 2022, Lexaria announced an exclusive commercial licensing agreement in Japan with Premier Wellness Science Co. Ltd. According to the terms of the agreement, Premier is purchasing the rights to DehydraTECH for the Japanese non-pharmaceutical market for various CBD and hemp-based products. For more details, check out our last medical sector roundup!
If that wasn’t enough, on June 8, 2022, Lexaria announced a five-year, non-exclusive licensing agreement with AnodGen Bioceuticals for DehydraTECH. This agreement will enable AnodGen to manufacture and distribute DehydraTECH processed cannabinoid APIs within Europe, including the UK, Australia, and New Zealand.
Most recently, on June 21, 2022, Lexarian announced the signing of two agreements with BevNology LLC., a company focused on providing formulation and commercialization services for beverages. The first agreement will allow Lexaria to use BevNology’s new facility, which is intended to significantly augment Lexaria’s production capacity.
The second agreement will allow BevNology to offer DehydraTECH products with APIs derived from hemp and CBD. However, for the Company’s powdered DehydraTECH formulations, this agreement is non-exclusive. Regarding Lexaria’s liquid DehydraTECH formulations, this agreement is non-exclusive in most areas of the world.
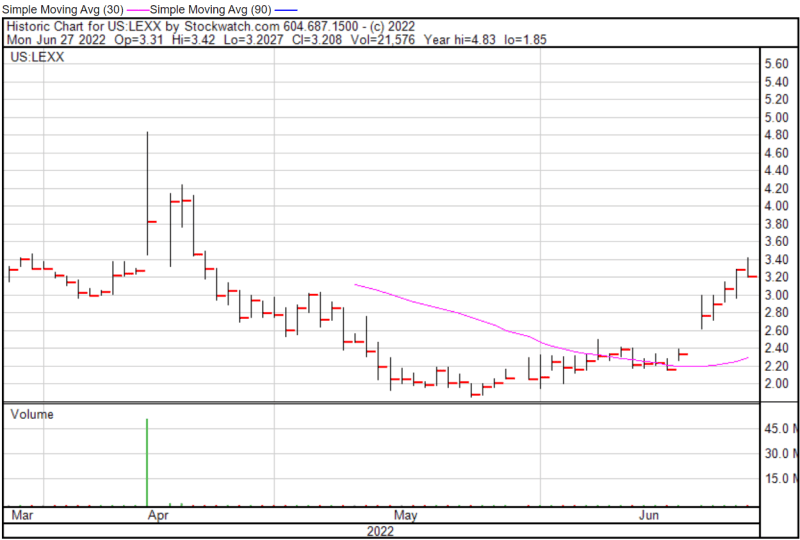
Lexaria’s share price opened at $3.34 on June 27, 2022, up from a previous close of $3.29. The Company’s shares were down -2.74% and were trading at $3.20 as of 1:52 PM EST on June 27, 2022.