Eye Don’t Believe It
Fill Disclosure: weekly roundups are intended to provide a quick introduction to various companies. They are not intended to be deep dives or opinion pieces.
Believe it or not, there is no evidence to suggest that sitting “too close to the TV” can damage children’s eyesight. Time to have a nice chat with our parents. Interestingly, if a child is sitting too close to the TV, this could indicative of a vision problem. Either that or your child is trying to talk to Eleven from Stranger Things. While sitting close to the TV can cause eye strain, there is no permanent damage.
Speaking of which, approximately 70% of millennials experience eye strain, according to SharpeVision. Prolonged periods of time spent focusing on screens, less time in daylight, and using devices in the dark all contribute to significant levels of eye strain. In a world filled with smartphones, tablets, and screens, this statistic may not be too surprising. As I write this, I am hunched over in a dark room like Gollum.
A Nielson audience report revealed that the average American spends more than 10 hours behind a screen. When it comes to millennials, this number reaches as high as 18 hours a day. With this in mind, cases of myopia have skyrocketed in the last decade, with myopia rates more than doubling in the US compared to the previous generation. Similarly, over 90% of children are leaving school myopic in Singapore.
Since this data is dated, let’s take a look at a more recent study published in Frontiers. This cross-sectional study was conducted in Shenzhen, China, and included 1,472,957 and 1,573,824 students in 2019 and 2020, respectively. Results found that the prevalence of myopia among students was 46.9% and 50.5% in 2019 and 2020, respectively.
In case you don’t know what myopia is, it is a common vision condition often referred to as “nearsightedness.” In other words, you can see objects near to you clearly, but struggle to see objects that are farther away. Personally, I struggle with nearsightedness and, as a result, I am required to wear glasses when I drive. So, why are cases of myopia increasing if eye strain doesn’t cause permanent damage?
One theory suggests that our eyes are gradually becoming more accustomed to only needing to see what is close to us, which is a screen more often than not. However, another theory argues that our eyes are not receiving enough natural sunlight due to prolonged periods of time spent indoors staring at, you guessed it, screens. What’s the solution for a generation of nearsighted, basement-dwelling millennials?
Well, cataract surgery is one of the most effective forms of refractive surgery, which is performed to improve the refractive state of the eye. By implanting a new lens, cataract surgery is able to correct almost any degree of hyperopia, myopia, and astigmatism at the time of surgery. That being said, let’s start this week’s roundup by looking at a company streamlining the cataract surgery procedure.
LENSAR Inc.

- $69.529M Market Capitalization
LENSAR Inc. (LNSR.Q) is a commercial-stage medical device company committed to designing, developing, and marketing advanced systems for the treatment of cataracts, with a focus on the management of astigmatism. For context, astigmatism refers to an imperfection in the curvature of the eye that causes blurred vision at all distances. More specifically, astigmatism impacts how the eyes intake light.
People with astigmatism may often find themselves squinting in an attempt to focus or reduce the glare they perceive from light sources. Astigmatism lights will appear as fuzzy halos, streaks, or glares, which is why night driving is especially difficult for those with astigmatism. With this in mind, LENSAR has developed ALLY, a compact system designed to deliver operational efficiencies and reduced overhead.
Latest News
On June 13, 2022, LENSAR announced that the U.S. Food and Drug Administration had provided clearance for the Company’s ALLY Adaptive Cataract Treatment System. As such, ALLY represents the first FDA-cleared platform enabling surgeons to complete laser-assisted cataract surgery in a single environment. Traditionally, laser-assisted surgery is split between a laser suite and an operating room.
“We are seeing an overwhelmingly positive response to the ALLY system. Over 125 surgeons have experienced ALLY firsthand, during demonstrations performed at the American Society of Cataract and Refractive Surgery Annual Meeting in April, and more recently at our home office,” said Nick Curtis, Chief Executive Officer of LENSAR.
However, ALLY is the first cataract surgery platform capable of automatically determining cataract density, fragmentation patterns, and energy settings, all in a single operating room or in-office surgical suite. In addition to having a small footprint, ALLY also incorporates advanced astigmatism management technology and can complete a procedure within half an hour.
Having received clearance, LENSAR plans to deliver the first ALLY systems to surgeons in the third quarter of 2022 through a controlled and targeted initial launch. However, the Company intends to make ALLY more widely available to cataract surgeons in 2023.
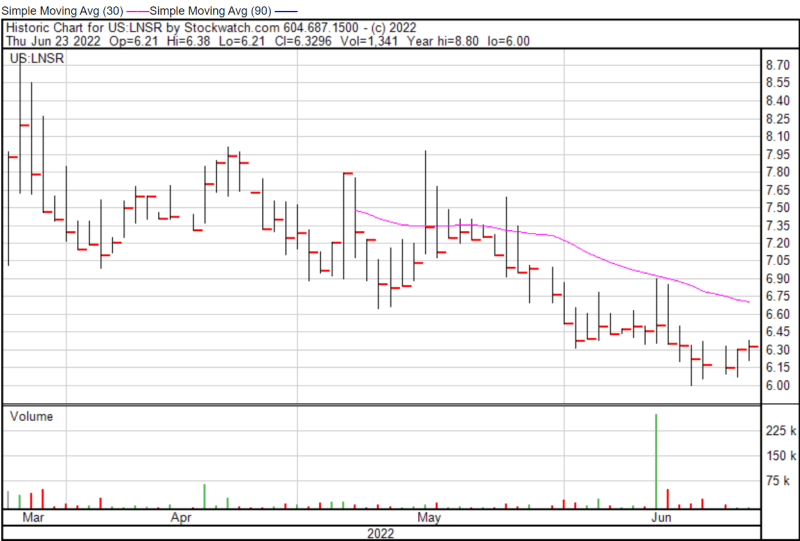
LENSAR’s share price opened at $6.36 on June 23, 2022, up from a previous close of $6.31. The Company’s shares were up 0.31% and were trading at $6.33 as of 10:22 AM EDT on June 23, 2022.
Lexaria Bioscience Corp.

- $18.032M Market Capitalization
Lexaria Bioscience Corp. (LEXX.Q) is a biotechnology company developing DehydraTECH™, the Company’s proprietary drug delivery technology. In addition to enhancing the speed and efficiency of orally-delivered drugs, DehydraTECH has demonstrated effectiveness in improving delivery through human skin for the potential development of topically-administered products such as patches.
Through various animal studies evaluating the quantity of drug delivery across the blood-brain-barrier (BBB) utilizing DehydraTECH technology, data suggests a gain of as much as 1,900%. With this in mind, Lexaria’s DehydraTECH has the potential to improve the delivery of certain central nervous system (CNS) targeted drugs against Alzheimer’s Disease, Parkinson’s Disease, and other CNS diseases.
Latest News
You are probably sick of seeing Lexaria in my roundups but bear with me. The Company appears to finally be shifting toward a revenue-generating model. Backed by numerous animal and human clinical studies, Lexaria has begun licensing its DehydraTECH technology to various companies, including Premier Wellness Science Co. Ltd. and AnodGen Bioceuticals.
Continuing with this trend, on June 21, 2022, Lexaria announced the signing of two agreements with BevNology LLC. BevNology is an Atlanta-based beverage development and advisory company offering formulation and commercialization services for “cutting-edge” beverage products. It goes without saying that DehydraTECH-infused beverages certainly fit this bill.
“BevNology’s formulation and production capabilities are class-leading and we are confident that our new relationship with our trusted partner will propel new and exciting growth opportunities for both companies,” said Chris Bunka, CEO of Lexaria.
To date, Lexaria’s DehydraTECH-CBD nano emulsification formulation and processing techniques have demonstrated beverage stability one year after production with less than 1% variability in CBD potency. CBD content was verified at 93.4% of target potency while also revealing significant microbial purity after 12 months.
According to the Company’s latest stability testing, DehyraTECH CBD-infused beverages were able to retain an average of 78% of CBD potency after a lengthy period of 25 months. Furthermore, Lexaria claims that the microbial purity and cleanliness of the product exceeded all requirements 25 months after bottling.
As for the agreements made with BevNology, the first agreement is a manufacturing operating agreement. This is intended to expand Lexaria’s production capabilities, allowing the Company to address a “growing list” of business-to-business (B2B) clients. These clients have a particular interest in Lexaria’s DehydraTECH enabled active ingredients for use in consumer packaged goods.
So far, Lexaria has moved and installed all required commercial DehydraTECH manufacturing equipment into BevNology’s new state-of-the-art processing facility. Lexaria’s second agreement with the company involves a commercial license agreement allowing BevNology to offer hemp-based DehyraTECH products under its own brand.
However, for powdered DehydraTECH formulations, this agreement is non-exclusive. Similarly, liquid DehyraTECH formulations are non-exclusive in most areas of the world, but limited exclusivity rights are available in the US alone, although the maintenance of these rights will require minimum fee payments. Furthermore, Lexaria will receive royalties from BevNology if this license is used.
Countries specifically excluded under this license include Japan, the Republic of Korea, and the People’s Republic of China. Seeing as Lexaria recently licensed DehyraTECH to Japan-based Premier, I can’t say I am too surprised. After all, Premier’s exclusive rights are subject to two previously issued licenses for DehydraTECH in Japan. As a result, Lexaria is unable to issue any further licenses in Japan.
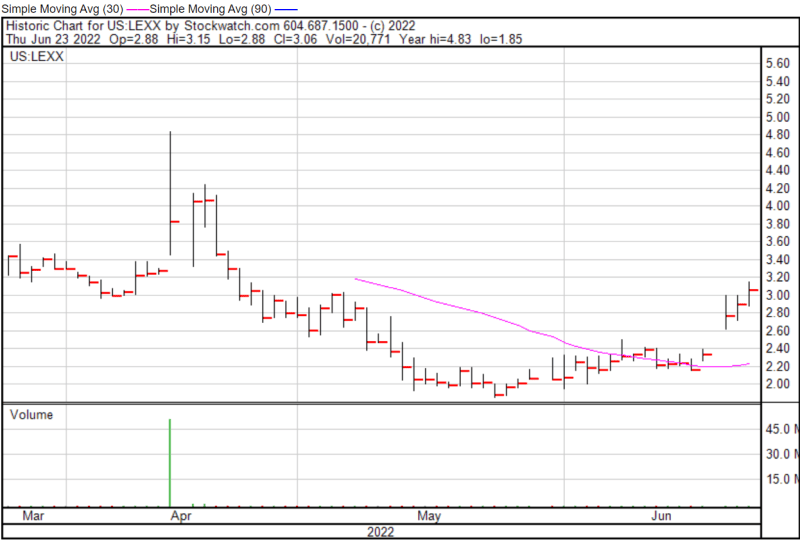
Lexaria’s share price opened at $2.98 on June 23, 2022, up from a previous close of $2.92. The Company’s shares were up 3.77% and were trading at $3.03 as of 12:21 PM EDT on June 23, 2022.
Axsome Therapeutics Inc.

- $895.013M Market Capitalization
Axsome Therapeutics Inc. (AXSM.Q) is a biopharmaceutical company developing novel therapies for central nervous system (CNS) diseases that have limited treatment options. That being said, AXS-05 represents the Company’s novel, oral, patent-protected, investigational NMDA receptor antagonist with multimodal activity under development for the treatment of Major Depressive Disorder (MDD) and other CNS disorders.
For context, MDD is a debilitating, chronic, biologically-based disorder characterized by poor mood, inability to feel pleasure, feelings of guilt and worthlessness, and low energy, among other emotional and physical symptoms. In severe cases, this can result in suicide. According to the World Health Organization, an estimated 7% of U.S. adults experience MDD each year.
Latest News
Not to put a damper on your Friday, but on June 6, 2022, Axsome recently announced the results of the SUPPORT survey evaluating 385 patients with depression. To provide some background, the SUPPORT survey refers to the “Studying the Impact of Patient Treatment Experiences on Patient Hope for the Future Major Depressive Pharmacotherapies” survey.
As the name suggests, this survey is intended to evaluate treatment experiences and expectations in patients taking antidepressants for MDD. Cutting to the chase, the survey revealed persistent and high levels of depressive symptoms despite treatment, significant interference with work and daily life, low levels of patient hope, and high levels of patient dissatisfaction with current treatments.
To be exact, 85% of survey participants reported high levels of dissatisfaction with currently available treatments despite being on antidepressants. In fact, a staggering 68% reported still experiencing moderate, severe, or very severe depressive symptoms with 48% of participants reporting at least one side effect. Needless to say, current MDD treatments, including antidepressants, fail to hit the mark.
There is some good news. Previously, on June 2, 2022, Axsome announced new data from the Company’s GEMINI Phase III trial in MDD. To summarize, the GEMINI trial assessed the safety and efficacy of Axsome’s AXS-05 versus placebo in a total of 327 patients with moderate to severe MDD. The primary endpoint of the study was the treatment effect on the MADRS score from baseline to week six.
“We are pleased to present these new data on AXS-05 at the 2022 annual meeting of the American Society of Clinical Psychopharmacology…These data support the efficacy of AXS-05 in patients with depression with a broad range of symptomatology, and further define its differentiated clinical profile,” said Herriot Tabuteau, MD, Chief Executive Officer of Axsome.
Put simply, the MADRS score is a ten-item diagnostic questionnaire used to measure the severity of depressive episodes in patients with mood disorders, such as MDD. In a secondary analysis of this trial, improvements in anhedonia symptoms in MDD were also assessed by the MADRS anhedonia subscale. For reference, anhedonia refers to the inability to feel pleasure, which is a common MDD symptom.
With this in mind, Axsome’s GEMINI trial demonstrated rapidly and significantly reduced anhedonic symptoms when using AXS-05 in treatment. Specifically, the change from baseline to week 6 on the MADRS anhedonia subscale was significantly greater with AXS-05 than with placebo.
In total, at week 6, a 50% improvement on the anhedonia subscale was achieved by 54% of patients treated with AXS-05 versus 36% of patients treated with a placebo. In addition to AXS-05, the Company has developed its FDA-approved Sunosi®, a dual-acting dopamine and norepinephrine reuptake inhibitor for the improvement of excessive daytime sleepiness (EDS). Where can I get some?
If you would like to know more about Sunosi, Axsome announced on June 22, 2022, that it will host a virtual event on June 28, 2022, at 9:00 AM EDT to provide an update on Sunosi for investors. During this event, the Company will provide a clinical overview of current and potential indications. Axsome’s senior management will also provide an overview of commercial activities and development plans.
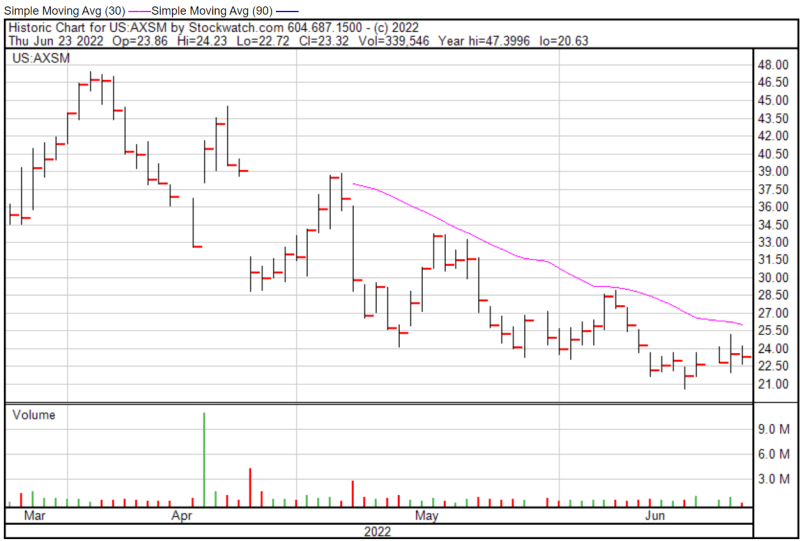
Axsome’s share price opened at $23.75 on June 23, 2022, up from a previous close of $23.57. The Company’s shares were down -0.70% and were trading at $23.41 as of 2:31 PM EDT on June 23, 2022.