Natural Talent

Albert Hofmann was a fun guy, pun intended. More importantly, the Swiss chemist is known for being the first person to synthesize, consume, and learn of the psychedelic effects of lysergic acid diethylamide (LSD). Truly a mad chemist. Furthermore, Hofmann was also the first person to isolate, synthesize, and name the principal psychedelic compounds of mushrooms, namely psilocybin and psilocin.
However, it is worth noting that while Hofmann was the first to synthesize and identify psilocybin and psilocin, psychedelics have been used for thousands of years. In fact, During his time as the director of Sandoz Lab’s natural products department in Mexico, Hofmann studied the hallucinogenic substances found in Mexican mushrooms and other plants used by the aboriginal people living there.
For example, the Mazatec people are an indigenous tribe hailing from the mountains of Oaxaca, Mexico. During their most sacred rituals, the Mazatec people utilize plants like psilocybin mushrooms to commune with spirits, divine information, heal ailments, and have a direct experience with the divine. I think I will wait until I am dead for a chance to speak to the big man upstairs. With this in mind, modern science has allowed us to harness the psychedelic potential of mushrooms.
As a result, many companies are now creating synthetic psychedelics for therapeutic use, which has a notable advantage over natural psychedelics, the advantage being predictability. Comparatively, natural psychedelics have undefined variabilities in psilocybin content between mushrooms and harvests, even when cultivated in controlled environments. With this in mind, the solution has traditionally been the development of synthetic psilocybin, whereby the potency levels can be controlled.
That being said, there are some advantages to natural psychedelics. For example, synthetic psychedelics are composed of single-compound molecules. On the contrary, natural psychedelics contain a host of secondary metabolites. Speaking of which, research has found that the presence of secondary metabolites may contribute to the therapeutic benefit of these substances, also known as the entourage effect. Not the show.
Put simply, the entourage effect refers to the synergistic interaction of two or more different molecules when co-administered, such as when a person consumes a natural extract. A study conducted in 2015 by Olga Zhuk and colleagues found that mushroom extracts were ten times more potent in serotonin than pure psilocin. Now, what if I told you there was a company that has unlocked the ability to produce safe and standardized natural psychedelic medicines?
Filament Health Corp.

- $20.503M Market Capitalization
Filament Health Corp. (FH.NE) is a clinical-stage natural psychedelics drug development company driven by the belief that safe, standardized, naturally-derived psychedelic medicines can improve lives. With this in mind, Filament is building a comprehensive platform to support the treatment of mental health conditions through administering natural psychedelic drug candidates during FDA authorized human clinical trials.
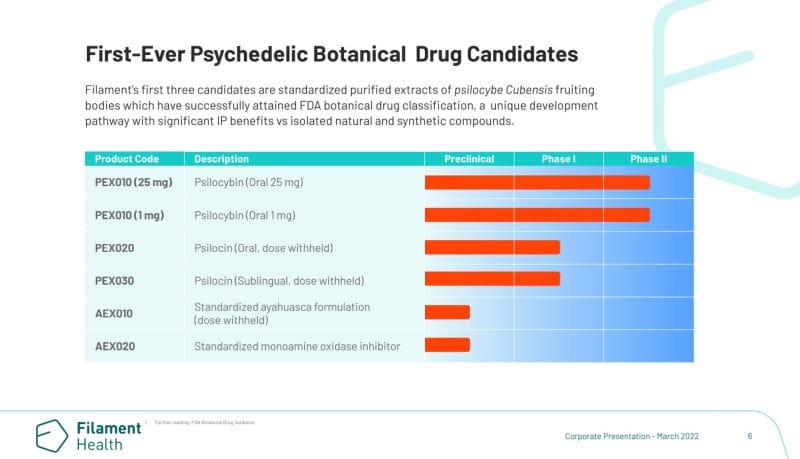
Here’s what sets Filament apart. The Company has also developed its own proprietary technology capable of producing stable, natural psilocin in addition to psilocybin. Furthermore, through Filament’s wholly-owned subsidiary Psilo Scientific Ltd., the Company operates one of the first GMP facilities in the world to also have a Health Canada Dealer’s License, providing Filament with a range of benefits including in-house trials and distribution of IP and drug candidates.
On that note, Filament recently received FDA authorization for a Phase I trial evaluating the efficacy of the Company’s drug candidates PEX020 and PEX030 against PEX010, Filament’s botanical psilocybin drug candidate. In addition to Filament’s Phase I trial, the Company received Health Canada approval on January 31, 2022, for a Phase II clinical trial using PEX010.
Latest News

On March 23, 2022, Filament announced that it has been issued a second patent by the Canadian Intellectual Property Office (CIPO) for the extraction and standardization of natural psilocybin and associated psychedelic compounds. The patent covers essential technology for transforming variable psychedelic raw materials into pharmaceutical-grade, standardized drug candidates.
“Two years ago, the conventional wisdom was that producing shelf-stable psilocin was impossible, Not only has Filament proved the contrary, but we have also had our innovations yet again validated by the patent office. The success rate for pending applications to issued patents in the pharmaceutical industry is 42.8%. Our success rate is 100%,” said Taran Grey, Director of Intellectual Property.
Filament’s latest successful patent issuance validates the Company’s IP strategy and sets the Company up for allowances of several pending patent applications covering additional elements of its proprietary technologies and compositions. If you’d like to know more about the Company’s second successful patent issuance, check out this article!
Additionally, on February 23, 2022, Filament announced that it has entered into a licensing agreement with ATMA Journey Centers. Under the agreement, Filament has licensed PEX010 (25 mg) to ATMA for use in clinical trials, including a Phase I psilocybin safety trial in healthy individuals enrolled in a psychedelic-assisted therapy training program. These trials are intended to provide therapists with an opportunity to gain in-depth psychedelic-assisted therapy practice.
“The selection of our natural psilocybin drug candidate for ATMA’s future clinical trials is a validation of both our product and the ease of working with Filament Health…ATMA is a leader in psychedelic-assisted psychotherapy and we are thrilled that they will be using our product,” said Filament Chief Executive Officer, Benjamin Lightburn.
The trial is expected to begin in mid-2022 and will be led by psychiatrist and Chief Medical Officer of ATMA, Dr. Ravinder Bains. In addition to providing therapists with experience, the trial’s primary objective will be to document the safety of synthetic psilocybin when administered to healthy participants in a controlled clinical setting. However, ATMA will also utilize Filament’s naturally-extractive botanical drug candidate, PEX010.
“Filament’s candidate was an attractive option because they are a licensed manufacturer and their drug candidate has received approval from Health Canada to enter into multiple human clinical trials. We are proud to support a thriving domestic psychedelics industry by selecting Filament’s natural, Canadian-made drug candidate for future studies,” stated David Harder, Co-Chief Executive Officer at ATMA.
For more details related to Filament’s licensing agreement with ATMA, check out this article! Overall, Filament possesses a valuable foothold in the Psychedelics Market, which is expected to reach USD$10.75 billion by 2027. Furthermore, there is a growing number of studies supporting the use of psychedelic compounds, including psilocybin and psilocin, to treat mental health issues.
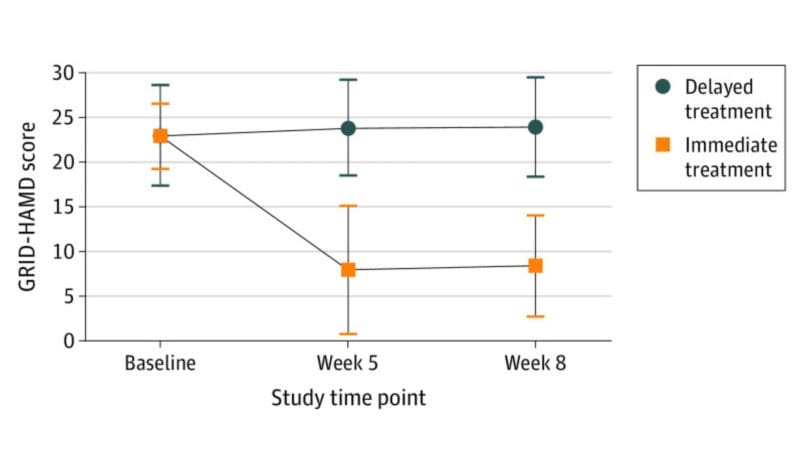
For example, a study published in the JAMA Psychiatry found that participants who received immediate psilocybin-assisted therapy compared with delayed treatment showed improvements in depression severity and in self-reported secondary outcomes through the 1-month follow-up. In other words, this randomized clinical trial demonstrated that psilocybin-assisted therapy was effective in producing large, rapid, and sustained antidepressant effects in patients with major depressive disorder (MDD).
Financials
Speaking of financials, according to Filament’s Q3 2021 Financial Results for the three and nine months ended September 30, 2021, and 2020, the Company had cash and cash equivalents of CAD$6,444,291 on September 30, 2021, compared to CAD$847,430 on September 30, 2020.
As of September 30, 2021, Filament had total assets and total liabilities of CAD$19,897,056 and CAD$1,248,127, respectively. For the same period in 2020, these numbers translate to CAD$881,561 and CAD$67,917, respectively.
Since going public, Filament’s expenses have increased substantially, which is to be expected especially for a clinical-stage company in the psychedelics sector. For the three and nine months ended September 30, 2021, the Company’s R&D expenses were CAD$176,403 and CAD$434,863, respectively.
According to Filament’s Q3 2021 Financial Results, the Company did not report revenue. However, shortly following the Company’s Q3 2021 results, Filament announced various licensing agreements with EntheoTech and Cybin. While the financial details of the Company’s agreement with Cybin remain undisclosed, we know that EntheoTech will pay $525,000 to Filament based on the achievement of certain milestones.
As of September 30, 2021, Filament had 164,756,869 issued and outstanding shares. Coming up, on February 22, 2022, 500,000 stock options at an exercise price of $0.30 will expire. Supported by multiple licensing agreements, unique IP, and various Health Canada approvals, Filament is a psychedelics company with some substance to it.
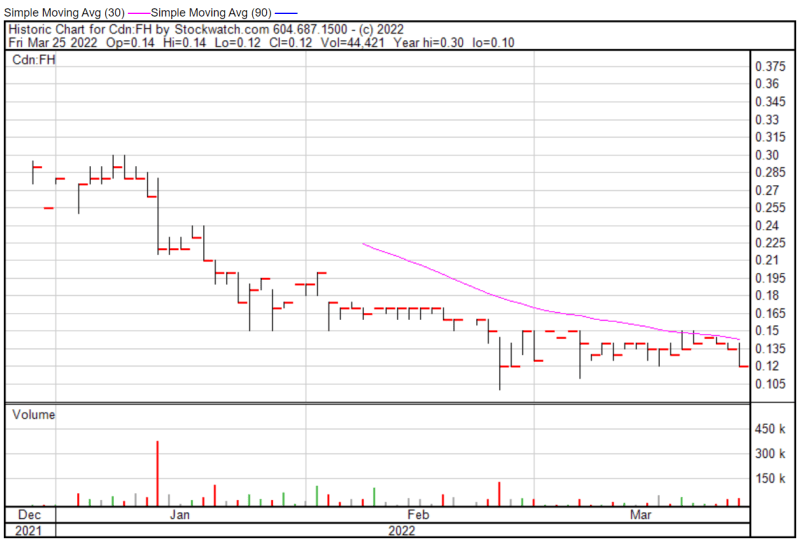
Filament’s share price opened at $0.14 on March 25, 2022, up from a previous close of $0.135. The Company’s shares were down -11.11% and were trading at $0.12 as of 2:57 PM EST.
Full Disclosure: Filament Health Corp. (FH.NE) is a marketing client of Equity Guru.