I’ll admit that I’m beginning to have my suspicions about the psychedelic sector.
The question I’ve started asking when I look at a new psychedelics company is where’s the evidence that they’re actually serious about getting products on shelves? How many startup drug companies at will never see the inside of a laboratory? Why? The prices are exorbitant and it’s easy to sit back and cozen investors into picking up their paper, which they can use to collect private placements and keep the lights on for another six months.
It’s hard to tell how much of the sector is like that. Maybe they had noble goals at one time, but time and circumstances took over and the stretch goal of late stage clinical trials with their $20 million minimum price tag could float away into the ether, leaving them looking for the next hot sector to pivot into. Like NFTs or something.
“The average cost of phase 1, 2, and 3 clinical trials across therapeutic areas is around $4, 13, and 20 million respectively. Pivotal (phase 3) studies for new drugs approved by the Food and Drug Administration (FDA) of the United States cost a median of $41,117 per patient.”
That really isn’t a lot of money if you can get enough of a bounce on the charts. That’s $37 million over a number of years required to go through clinicals. And that’s if they pass each trial. Naturally, that’s after pre-clinicals—which bump the price tag considerably—but not exactly out of the realm of possibility.
Then there’s the high price of failure in this sector. If you’ll remember back to your days in the small desks in public school science class, the scientific method includes a good amount of failure. You take all the data, produce your hypothesis, and give it up to rigorous testing and a lot of the time that testing fails. If that’s adding baking soda, sand, food colouring and vinegar to make a volcano for an A-grade and you accidentally use flour instead of baking soda (or something) then the cost of failure is negligible. A few bucks.
If it’s a botched variable in a multi-variable study that produces a result that’s not statistically significant, it might take you three or four iterations of the study to find the variable, and that can get costly.
How costly?
“Many recent studies have focused on how much it costs to bring a new drug to market in the light of increasing drug prices. Estimates range from $314 million to $2.8 billion. Most of this was based on proprietary data and was, therefore, not verifiable independently, attracting much criticism,” according to Dr. Liji Thomas, writing for news-medical.net.
Those numbers factor in more than just the science, though. There’s licensing, packaging, distribution, etc. But the science is a big stumbling block for a lot of companies. If you fail at that $20 million mark, you’re going to have to pay another $20 million. That’s why if you’re looking to get into the psilocybin sector as an investor, you want to find a company that’s got a solid deal with a university or some other research-based entity to offset the cost.
Companies like Filament Health (FH.E), for example.
Sector strong excellence
Filament is a clinical-stage psychedelic drug development company working on researching and developing new medicines using psilocybin and other compounds. Their extensive intellectual property portfolio gives them the unique opportunity to discover, develop and produce delivery systems for natural psychedelic medicines for clinical development.
What’s most interesting is that this company skipped the humming and hawing phase and went straight to getting the work done. They received the Health Canada nod early to continue with their phase 2 clinical trial using their standardized psilocybin drug candidate PEX010.
The trial will study the safety and use-value of low doses of psilocybin in otherwise health subjects with persistent depressive disorder. It’ll be led by Rotem Petranker, the director for the Canadian Centre for Psychedelic Science, and Dr. Normal Farb at the University of Toronto. The trial has also received financing from the Nikean Foundation.
“The Filament Health team has been instrumental in getting this trial up and running…I am excited to work with Filament, whose commitment to professionalism and Open Science has been unwavering. Filament’s product allows us to closely approximate the conditions under which people microdose in the real world, and I expect the results from this study to be very informative,” said Rotem Petranker, Director of the Canadian Centre for Psychedelic Science and the study’s Principal Investigator.
The trial is expected to begin dosing in Q1, 2022, and will include 100 healthy subjects with symptoms of the disorder. The trial will follow the effects of PEX010 at one milligram. Also, the drug recently received the nod from the FDA to enter into phase 1 clinicals at the University of California, San Francisco.
Let’s add onto that.
The company entered into a licensing agreement earlier this month with private pharmaceutical company, Cybin Therapeutics, wherein they’ll license their experimental botanical psilocybin drug candidate PEX010 to Cybin for two Phase II clinical trials to address depression and alcohol use disorder, with two more trials planned for 2022.
“Since 2000, there have been 327 clinical trials globally investigating the use of psychedelics in mental healthcare. There are currently 38 Phase 1 and 26 Phase 2 trials for the use of psilocybin in mental health treatment. Results have been promising, with the largest randomized-controlled double-blind psilocybin study showing rapid and sustained positive response to psilocybin and its concurrent therapy,” from their blog.
From their activity in the first month of 2022 alone, it should be obvious that Filament is not one of those companies inclined to coasting in this sector.
The Numbers
Admittedly, the numbers here could be better, but that’s fairly common in this nascent sector.
Third quarter and postperiod operational highlights
- FDA (Food and Drug Administration) authorization of phase I clinical trial using naturally sourced psychedelic substances and direct administration of psilocin (both orally and sublingually) for the first time in FDA history;
- First and only patent granted for the extraction and standardization of natural psilocybin;
- Over 20 patent applications to date, including three international patent co-operation treaty (PCT) applications covering extraction, standardization, purification and delivery of botanical drug candidates;
- Completed industry-first export of current good manufacturing practices (cGMP)-quality natural psychedelic substances to the United States;
- Psilo Scientific Ltd., Filament’s wholly owned subsidiary received an amendment to the Health Canada dealer’s licence for its facility, allowing the possession, production and delivery of all controlled natural psychedelic substances;
- Produced pharmaceutical-grade batches of natural psilocybin extracts, including leading drug candidates which will be entered into coming FDA clinical trials.
Third quarter and postperiod financial and capital markets highlights
- Uplisted to the OTCQB Market under the symbol FLHLF on Oct. 12, 2021;
- Received Depository Trust Company (DTC) eligibility on Oct. 12, 2021;
- Listed on the Frankfurt Stock Exchange under the symbol 7QS on Oct. 15, 2021;
- Cash and cash equivalents of $6.4-million as of Sept. 30, 2021.
You’re not going to find any revenue in this chart because there isn’t any yet. They need to get a product through the scientific rigamarole before they can even think about branding and marketing, let alone finding distribution, and right now they’re staring down the FDA. There’s still room for failure here, but the probability of such isn’t strong.
Normally I don’t pull out a chart in these longer form stories. I leave that to our resident chart wizard Vishal Toora, who you should likely go to for deeper, pro-forma analysis than the puddle deep technicals I can provide, but what’s obvious is obvious.
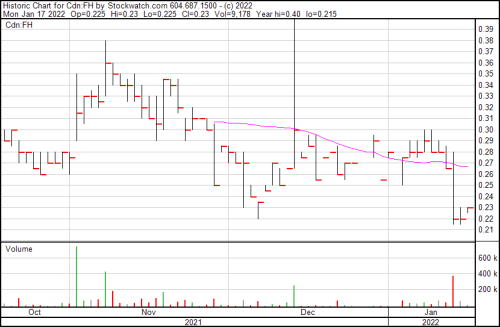
This company hasn’t been getting the love it deserves just yet—and there’s solid reasons why not—but if you’re looking for a company with irons in the fire and a long term trajectory that could theoretically include a handful of doubles and triples in the future as trials end and products get on shelves, you could do worse than Filament.
That’s solid good news for you, because this company presently has a market cap approximating $36 million, but it’s doubtful it’s going to stay there for long.
—Joseph Morton
Full disclosure: Filament Health is an equity guru marketing client.