Cancer is a bitch. Even if you haven’t had to battle against cancer yourself, you likely know someone who has. Unfortunately for me, that person was my mom; however, before this turns into a sob story let me assure you that my mom is alive and well after kicking cancer’s ass. My mom was diagnosed with breast cancer when I was still going through my angsty teenager phase. Being young, dumb, and ignorant, I ended up causing a lot of trouble for my mom. I was the kind of kid who would create problems for myself just so I could justify doing stupid shit like smoking cigarettes, drinking excessively, hanging out in places I shouldn’t have, and skipping classes. When my mom was diagnosed with breast cancer, my lofty ambition to be like John Bender from The Breakfast Club felt so childish and insignificant. It’s like a switch flipped and I finally realized that even if I thought I was invincible, the same couldn’t be said for the people around me.
Thankfully, my mom’s breast cancer was caught early and she was able to remove the cancerous tumor via a mastectomy, a procedure to remove one or both breasts, either partially or completely. Next to lung cancer, breast cancer is the second leading cause of cancer death in women. Statistically speaking, the odds of a women dying from breast cancer are around 1 in 39, or 2.6%. In 2019, breast cancer accounted for 7% of all cancer related deaths, claiming the lives of 42,281 females in the United States according to the Centers for Disease Control and Prevention (CDC). Keep in mind, breast cancer is just one of more than 100 different types of cancer, including acute myeloid leukemia (AML).
In particular, AML is a type cancer that starts in blood stem cells, which are produced by the bone marrow. These stem cells are basic cells that develop into different types of cells such as red blood cells, white blood cells, and platelets. However, AML promotes the production of immature blood cells, eventually overcrowding and preventing the function of normal blood cells. There are various treatment options for AML, including chemotherapy, targeted therapy, and an allogeneic stem cell transplant (allo-HSCT). With this in mind, treosulfan is Medexus’ innovative, orphan-designated agent developed for use as part of a conditioning treatment for patients undergoing allo-HSCT.

Who is Medexus Pharmaceuticals?
Medexus Pharmaceuticals (MDP.T) is an innovative and rare disease pharmaceutical company with a strong North American commercial platform. The Company is focused on the therapeutic areas of rheumatology, auto-immune disease, specialty oncology and pediatrics. Medexus is led by its founder, CEO and President, Ken d’Entremont, a man who has supported the Company since its inception in 2000. Prior to founding Medexus, Mr. d’Entremont was the general manager and vice president of business development at Sanofi, where he led the in-licensing initiatives for Sanofi Canada, a global biopharmaceutical company focused on the development of healthcare solution from prevention to treatment. Overall, Mr. d’Entremont is responsible for leading Medexus to achieve profitability in just its third year of operations.
Currently, Medexus is in the process of pursuing commercialization for treosulfan in the United States. As previously mentioned, treosulfan is a medication intended for use as part of a conditioning treatment for patients undergoing allo-HSCT. Treosulfan has been demonstrated to have a 26% improvement in overall survival over 24 months for leukemia patients. Furthermore, in a pivotal Phase 3 Trial, treosulfan demonstrated an increased rate of event-free survival 2 years after allogeneic transplantation, compared to the current standard of care. With this in mind, Medexus’ treosulfan is associated with clinically meaningful benefits for higher-risk allo-HSCT patients. However, it hasn’t been all sunshine and rainbows for the Company. Despite being supported by results from a Phase 3 Trial and more than 100 publications, Medexus’ New Drug Application (NDA) for treosulfan has not yet been approved by the U.S. Food and Drug Administration (FDA.
“…we remain optimistic for a future, albeit delayed, approval of treosulfan in the United States, complete with Orphan Drug Designation. The current standard of care is not suitable for numerous at-risk groups, due to the high toxicity effects, and treosulfan has demonstrated excellent survival data among those groups…,” said Ken d’Entremont.
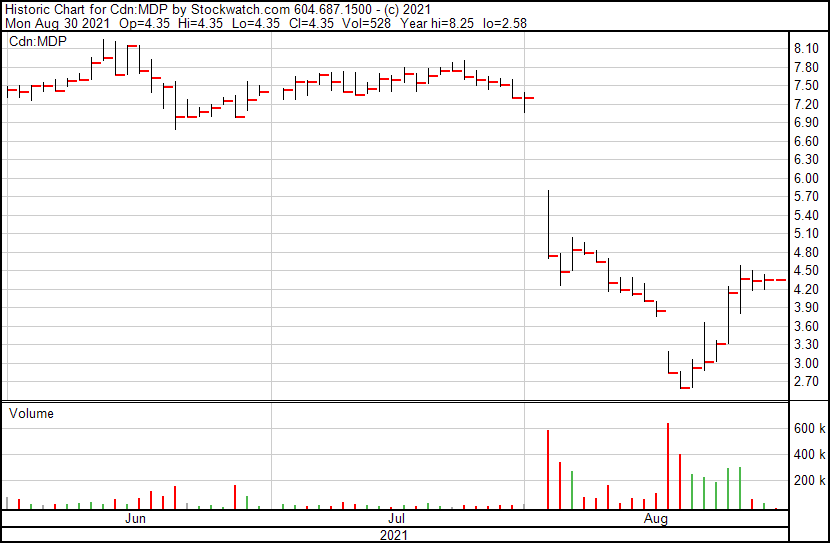
On August 3, 2021, Medexus announced that it had received a Complete Response Letter (CRL) from the FDA in response to its proposed NDA for treosulfan. In the CRL, the FDA cited that it could not approve Medexus’ NDA in its current form. Considering treosulfan has already received marketing approval in both Europe and Canada, this news caught many off guard. However, this does not speak to Medexus’ commercial success as a whole. Although the Company’s IXINITY® sales were reduced due to inventory issues, unit demand for IXINITY® grew 25.3% year-over-year. Similarly, the Company’s Rupall™ (not the drag race) anti-histamine medication saw unit demand increase 44% year-over-year, according to Medexus’ Financial and Operational Results.
Speaking of IXINITY®, on August 12, 2021, Medexus announced that it had completed enrollment in its Phase 4 Clinical Trial of IXINITY®, targeting label expansion for pediatric hemophilia B. For context, hemophilia B is a hereditary bleeding disorder characterized by a deficiency of clotting factor XI, a blood clotting factor that protects the body by sealing off damaged blood vessels and preventing further blood loss. In other words, if you have a cut, clotting factor XI will make sure that bad boy stops bleeding…eventually. However, individuals with hemophilia B have a deficiency of blood clotting factor XI, making them more susceptible to bruising and bleeding.
“Previously reported and pooled data from Phase 3 clinical trials demonstrated IXINITY® to be safe and well tolerated in preventing and controlling bleeding episodes in treated children under the age of 12 with hemophilia B. If approved, we expect to be well positioned to commercialize quickly with the infrastructure we already have in place for the adult market,” commented Ken d’Entremont.
With this in mind, IXINITY® is an FDA approved intravenous recombinant factor XI therapeutic for use in patients 12 years or older with hemophilia B. Once completed, Medexus’ Phase 4 Clinical Trial will assist the Company in pursuing label expansion for IXINITY®. In particular, Medexus hopes to extend the reach of IXINITY® to include the U.S. pediatric populations below 12 years of age. According to the World Federation of Hemophilia, approximately 1 in 3 patients treated for hemophilia B are 12 years of age or younger, indicating a prime market for the Company’s IXINITY®.
Is Medexus Worth Your Time?
With a diversified portfolio of commercial and late-stage pharmaceutical products, Medexus has a reliable growth profile. Looking forward, the Company plans to license and acquire additional products to leverage its North American sales force and infrastructure. Additionally, Medexus could very well obtain FDA approval to commercialize treosulfan for allo-HSCT in the U.S. in the near future. Keep in mind, the Acute Myeloid Leukemia Therapeutics Market is expected to reach $18.3 billion by 2026, expanding at a respectable CAGR of 7.2% between 2021 and 2026. Moreover, allo-HSCT is currently the most effective treatment in eligible patients with AML or high-risk myelodysplastic syndromes (MDS). Furthermore, if Medexus is able to expand IXINITY®’s label to include pediatric indication, this could increase the Company’s addressable market size for IXINITY® by 30%. As a whole, the Global Hemophilia Market is expected to reach USD$18.1 billion by 2027, growing at a CAGR of 5.5%, according to Grand View Research. With a market capitalization of $83.375 million, Medexus still has plenty of room to grow and treosulfan could very well become the Company’s golden goose egg if approved for commercialization in the U.S.