Mind Medicine (MNMD.Q, MMED.NE) announced that they have agreed to partner with Forian (FORA.Q), who provide evidence-based support for clinical and commercial decision making.
Forian and MindMed will collaborate to create best practices for using real-world evidence information to improve their treatments. In their press release, MMED says this is to improve patient outcomes, and that is certainly true, but it is also important to make sure their treatments do well on the metrics used by the FDA when deciding whether to grant approval of a given treatment.
Forian has experience working with governments and government agencies, as they currently serve nine state governments, evaluating the therapeutic value and reduction in healthcare costs associated with cannabinoid-based therapeutics as alternatives to traditional ethical pharmaceutical products and healthcare services, as well as monitoring social equity issues related to the distribution of licenses for the state governments.
Experience working on cannabis licensing could provide them with useful experience that will help to navigate the regulatory landscape of psychedelics.
Forian uses innovative new technologies driven by data science and built upon proprietary data. They use a ‘Data Factory’ which “transforms raw, unstructured, disparate data into a cleansed, connected, and normalized data lake, supporting efficient and scalable analytics.”
“Our goal is to use real-world healthcare data and deep digital phenotyping to achieve in-depth, precise and personalized characterizations of individuals with generalized anxiety disorders and other conditions of interest for our drug development programs,” stated MindMed’s Chief Medical Officer Dan Karlin. “By fully acknowledging each person’s physiology, environment, and behavioral stressors, we ultimately aim for integrated digital deep diagnoses to drive precision psychiatry for drug development and clinical treatment across our target therapeutic areas.”
MindMed already has one drug candidate in Phase 2b, so this partnership is likely looking forward towards a Phase 3 study. Phase 3 studies often involve more raw information that needs to be processed and analyzed than earlier studies. Phase 3 studies can involve as many as 3,000 participants, so analyzing data manually time-consuming.
Beyond their LSD molecule in Phase 2b, MindMed also have 11 other psychedelic candidates currently in clinical trials, with four in Phase 1 studies and seven in Phase 2a. These compounds range from LSD to Mescaline to DMT and target a variety of health issues, such as opioid withdrawal, depression, adult ADHD, cluster headaches, and more. Analyzing all the data from these studies could help MMED optimize the treatments and see potential they may have otherwise missed.
“Forian understands the power our real-world evidence and unique data sets add to our healthcare and pharmaceutical partners,” commented Dan Barton, CEO of Forian. “We are thrilled to collaborate with MindMed to help them deliver insights to improve the health of psychiatric patients.”
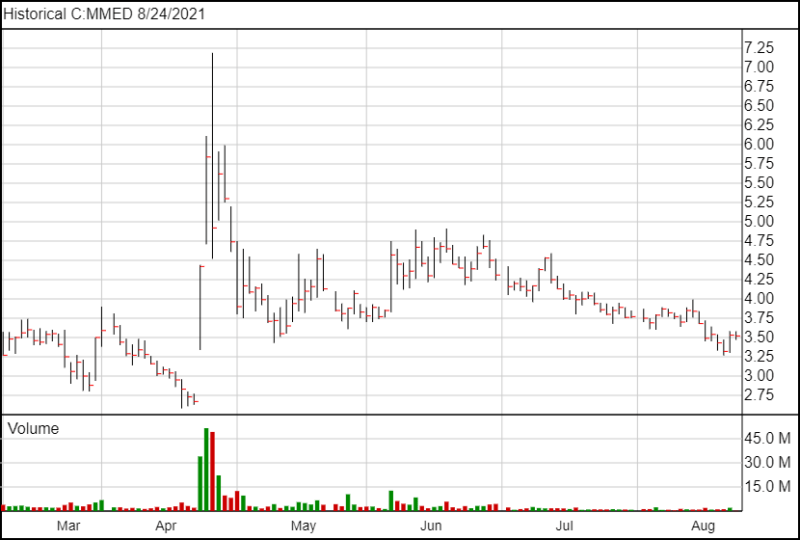
Following the news, MindMed shares are down 1 cent and are currently trading at $3.52